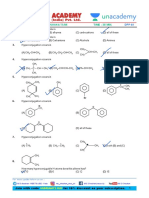
Professional Documents
Culture Documents
Solutions DPP 3
Uploaded by
Tech. Videcious0 ratings0% found this document useful (0 votes)
16 views3 pagesSolutions DPP 3
Chemistry by Dr. Sunny Garg
SCO 26, Sector 15 panchkula
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentSolutions DPP 3
Chemistry by Dr. Sunny Garg
SCO 26, Sector 15 panchkula
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
16 views3 pagesSolutions DPP 3
Uploaded by
Tech. VideciousSolutions DPP 3
Chemistry by Dr. Sunny Garg
SCO 26, Sector 15 panchkula
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 3
1
PATHSHALA JEE Dropper
DPP-03
Solutions
1. Which of the following is not correct for 5. All form ideal solution except
ideal solution (A) C2H5Br and C2H5I
(A) Raoult's law is obeyed for entire (B) C6H5Cl and C6H5Br
concentration range and temperatures (C) C6H6 and C6H5CH3
(B) Smix = 0 (D) C2H5I and C2H5OH
(C) Vmix = 0
(D) Hmix = 0 6. Total vapour pressure of mixture of 1 mol A
( PA = 150 torr) and 2 mol B ( PB = 240 torr)
2. Azeotropic mixture of water and HCl boils is 200 mm. In this case
at 381.5 K. By distilling the mixture it is (A) There is positive deviation from
possible to obtain Raoult's law
(A) Pure HCl only (B) There is negative deviation from
(B) Pure water only Raoult's law
(C) Neither HCl nor water (C) There is no deviation from Raoult's law
(D) Both water and HCl in pure state (D) Molecular masses of A and B are also
required for predicting deviation
3. An azeotropic solution of two liquids has
boiling point lower than either when it 7. Which of the following will form an ideal
solution?
(A) shows a negative deviation from
Raoult's law (A) C2H5OH and water
(B) shows a positive deviation from (B) HNO3 and water
Raoult's law (C) CHCl3 and CH3COCH3
(C) shows no deviation from Raoult's law (D) C6H6 and C6H5CH3
(D) is saturated
8. Which of the following shows positive
4. 100 ml of liquid A and 25 ml of liquid B is deviation from Raoult's law?
mixed to give a solution which does not (A) C6H6 and C6H5CH3
obey Raoult's law. The volume of the (B) C6H6 and CCl4
solution (C) CHCl3 and C2H5OH
(A) will be 125 ml (D) CHCl3 and CH3COCH3
(B) can be > or < than 125 ml
(C) can be greater than, equal to or less 9. Which of the following shows negative
than 125 ml deviation from Raoult's law?
(D) will be less than 125 ml (A) CHCl3 and acetone
(B) CHCl3 and C2H5OH
(C) C6H5CH3 and C6H6
(D) C6H6 and CCl4
2
10. Which of the following solution pairs can be 12. The diagram given below represents boiling
separated by fractional distillation ? point composition diagram of solution of
(A) Water HNO3 component A and B, what is true among the
(B) Water-HCl following?
(C) Benzene-toluene
(D) C2H5OH-water
11. The diagram given below is a vapour
pressure composition diagram for a binary
solution of A and B in the solution, A – B
interactions are-
(A) The solution shows negative deviation
(B) A-B-interactions are stronger than A-A
and B-B
(C) The solution is ideal solution
(D) The solution shows positive deviation.
(A) Similar to A – A and B – B interactions
(B) Greater than A – A and B – B
interactions
(C) Smaller than A – A and B – B
interactions
(D) Unpredictable
3
ANSWER KEY
1. (B)
2. (C)
3. (B)
4. (B)
5. (D)
6. (B)
7. (D)
8. (C)
9. (A)
10. (C)
11. (C)
12. (D)
You might also like
- Puc II Chem Mcqs 2024Document113 pagesPuc II Chem Mcqs 2024geethaathradyNo ratings yet
- Ncert Exemplar ChemistryDocument22 pagesNcert Exemplar Chemistrysheetal10swetaNo ratings yet
- 12TH Class Chapter Wise QP 2022-23Document146 pages12TH Class Chapter Wise QP 2022-23Aaghash A SNo ratings yet
- Adobe Scan Jul 02, 2023Document6 pagesAdobe Scan Jul 02, 2023VILLAIN EX.No ratings yet
- Solution Objectives TestDocument4 pagesSolution Objectives TestBhavyNo ratings yet
- CH1 Soution HHW Worksheet1Document6 pagesCH1 Soution HHW Worksheet1Aaditya SharmaNo ratings yet
- Solution PDFDocument5 pagesSolution PDFGourab SahaNo ratings yet
- Solutions: 1 Year Chemistry N0tes NewDocument9 pagesSolutions: 1 Year Chemistry N0tes NewAboahmed AliNo ratings yet
- 12 Solution Dpp-2Document10 pages12 Solution Dpp-2Ananya SharmaNo ratings yet
- solutions answersDocument38 pagessolutions answersjyotirmayeekansraliNo ratings yet
- Chemistry CH 9 McqsDocument2 pagesChemistry CH 9 Mcqsshahid abbasNo ratings yet
- 9 CHAPTER SOLUTIONS MCQsDocument9 pages9 CHAPTER SOLUTIONS MCQsNouman RanaNo ratings yet
- 01 Solutions Questions For PracticeDocument19 pages01 Solutions Questions For PracticeharshalNo ratings yet
- SolutionsDocument5 pagesSolutionsPranav ShinojNo ratings yet
- Basara Gnanasaraswathi Campus Kakatiya HillsDocument8 pagesBasara Gnanasaraswathi Campus Kakatiya HillsSree Charan SohanNo ratings yet
- Adobe Scan 23 Dec 2022Document7 pagesAdobe Scan 23 Dec 2022GAURAV kumarNo ratings yet
- Chap - 9 SolutionsDocument9 pagesChap - 9 SolutionsKamal KishoreNo ratings yet
- Solution SolDocument5 pagesSolution Solno nameNo ratings yet
- 2.MCQ SolutionDocument26 pages2.MCQ SolutionShaurya YadavNo ratings yet
- Alkyl and Aryl Halides SheetDocument11 pagesAlkyl and Aryl Halides SheetRajeev GangwarNo ratings yet
- Day-5 SolutionsDocument5 pagesDay-5 SolutionspriyanshuNo ratings yet
- 1 MS SolutionDocument11 pages1 MS SolutionsachinNo ratings yet
- Solutions (MCQ, Assertion & Case Base)Document16 pagesSolutions (MCQ, Assertion & Case Base)ANKUSH HOODANo ratings yet
- 12th Class Chapter Wise QP 2022-23Document146 pages12th Class Chapter Wise QP 2022-23Avi KedarrNo ratings yet
- CH 9. Alkyl HalidesDocument47 pagesCH 9. Alkyl HalidesSajag GargNo ratings yet
- Unit Solution 70 MarksDocument5 pagesUnit Solution 70 MarksअनंतNo ratings yet
- ExerciseDocument42 pagesExerciseMoneyNo ratings yet
- 1 QP SolutionDocument6 pages1 QP SolutionsachinNo ratings yet
- Liquid Solution: Henry's Law, Osmotic Pressure, and Colligative PropertiesDocument29 pagesLiquid Solution: Henry's Law, Osmotic Pressure, and Colligative PropertiesSumant KumarNo ratings yet
- Class 12 Preboard Chemistry Answer KeyDocument7 pagesClass 12 Preboard Chemistry Answer KeyDiksha TNo ratings yet
- Class 12 Chemistry Line by Line 2024-25 Ch-1.SolutionsDocument39 pagesClass 12 Chemistry Line by Line 2024-25 Ch-1.SolutionsAbhinav VermaNo ratings yet
- Sample Paper - 6Document8 pagesSample Paper - 6rajneesh kumarNo ratings yet
- Vacation HWDocument67 pagesVacation HWMohammed NayeemNo ratings yet
- Chem 1212 Exam KeyDocument6 pagesChem 1212 Exam KeyChris HeNo ratings yet
- Liquid SolutionDocument9 pagesLiquid Solutionpurri4lifeNo ratings yet
- Ms Chouhan & Team: Organic ChemistryDocument16 pagesMs Chouhan & Team: Organic ChemistryPrithviraj GhoshNo ratings yet
- Sheet - 01 - Biomolecules-ExtractedDocument34 pagesSheet - 01 - Biomolecules-Extractedbaibhav singhNo ratings yet
- Mole Concept-1 JEE Main and Advanced PDFDocument6 pagesMole Concept-1 JEE Main and Advanced PDFAryan Jaiswal100% (1)
- One Markc Combined Board QuestionsDocument19 pagesOne Markc Combined Board Questionssyedasifbasha1990No ratings yet
- Solution Colligative Properties ExplainedDocument31 pagesSolution Colligative Properties ExplaineddislikeNo ratings yet
- Vidyashram Public School Pre-board Examination (2021-22) Chemistry Class 12thDocument8 pagesVidyashram Public School Pre-board Examination (2021-22) Chemistry Class 12thKhushi BNo ratings yet
- Sample Paper 3: ChemistryDocument13 pagesSample Paper 3: ChemistryPr SathishNo ratings yet
- Alkyl Halides Solutions (-2) ChemDocument45 pagesAlkyl Halides Solutions (-2) ChemChauhan RonakNo ratings yet
- Chemistry by Mukesh SharmaDocument13 pagesChemistry by Mukesh Sharmaaleena'No ratings yet
- Weight) : Following Colligative Property?Document6 pagesWeight) : Following Colligative Property?Sanjukta DashNo ratings yet
- Liquid Solution Boiling PointsDocument3 pagesLiquid Solution Boiling PointsAbhishek GumwantNo ratings yet
- Ionic EquilibriumDocument11 pagesIonic EquilibriumrashidNo ratings yet
- Chemistry Ch-9 Part-IDocument9 pagesChemistry Ch-9 Part-IDr. Abdul Haq BalochNo ratings yet
- OrganicDocument9 pagesOrganicjitesh100kushwahaNo ratings yet
- Solutions+ +Top+Practice+Problems++ (19!04!2021)Document57 pagesSolutions+ +Top+Practice+Problems++ (19!04!2021)Ritheesh NagarajanNo ratings yet
- Liquid Solution - Practice SheetDocument7 pagesLiquid Solution - Practice SheetYashvik GuptaNo ratings yet
- Class 12 Chemistry Test on Colligative Properties and SolutionsDocument8 pagesClass 12 Chemistry Test on Colligative Properties and SolutionsOviya VNo ratings yet
- Chapter 6 Hydrocarbons and Alkenes SolutionsDocument26 pagesChapter 6 Hydrocarbons and Alkenes SolutionsjanNo ratings yet
- Class 12 Chemistry Half Yearly VMCDocument7 pagesClass 12 Chemistry Half Yearly VMCno accountNo ratings yet
- Highest Selection in IIT-JEE since 2006Document13 pagesHighest Selection in IIT-JEE since 2006adityaNo ratings yet
- Halogen Derivatives SheetDocument6 pagesHalogen Derivatives SheetRajeev GangwarNo ratings yet
- 1 QP SolutionDocument6 pages1 QP Solution27122005adityagargNo ratings yet
- Adobe Scan Feb 28, 2023Document11 pagesAdobe Scan Feb 28, 2023Vikram NeelmegamNo ratings yet
- Science-SQP 2 Term2Document13 pagesScience-SQP 2 Term2Srivatsan BalajiNo ratings yet
- Ball Mill Operating Manual 266795 Manual InstrDocument48 pagesBall Mill Operating Manual 266795 Manual InstrHarish Chandra SinghNo ratings yet
- Em+2 Qrtly Exam Chem Imp Questions-2023Document6 pagesEm+2 Qrtly Exam Chem Imp Questions-2023Azees AzeesNo ratings yet
- 3M Half Facepiece Respirator 6000 Series User InstructionsDocument2 pages3M Half Facepiece Respirator 6000 Series User InstructionsPaola Millare - MuharraniNo ratings yet
- Chemistry Paper 2 TZ2 SL Markscheme May 2018 EveDocument13 pagesChemistry Paper 2 TZ2 SL Markscheme May 2018 EveJustNo ratings yet
- Grouting in PostDocument7 pagesGrouting in PostDeven PatleNo ratings yet
- (2011) Bernauer's Bands - Alexander ShtukenbergDocument14 pages(2011) Bernauer's Bands - Alexander ShtukenbergwalterNo ratings yet
- Biology Practice EOCDocument76 pagesBiology Practice EOCHeri SetiadiNo ratings yet
- ReinoldsDocument1 pageReinoldsJalvareztejadaNo ratings yet
- Durapore 33 MM MillexDocument4 pagesDurapore 33 MM MillexSigit SadewoNo ratings yet
- AS/A2 - Chemistry: 2021-23 CurriculumDocument80 pagesAS/A2 - Chemistry: 2021-23 CurriculumenderothNo ratings yet
- Drugs - How To Make Cocaine Synthetically)Document4 pagesDrugs - How To Make Cocaine Synthetically)api-2618198977% (22)
- Passing Package, Haloalkanes and HaloarenesDocument8 pagesPassing Package, Haloalkanes and HaloarenesShalem JeldiNo ratings yet
- F Block Elements PDFDocument3 pagesF Block Elements PDFRamya PatelNo ratings yet
- Tensile Properties of Ground Coffee Waste Reinforced Polyethylene CompositeDocument4 pagesTensile Properties of Ground Coffee Waste Reinforced Polyethylene CompositemhmmdzulvaNo ratings yet
- MMSB Exercise SolutionsDocument49 pagesMMSB Exercise SolutionsYolanda Winarny Eviphanie HutabaratNo ratings yet
- Cyclic Steam Stimulation Thermal EOR ProcessDocument48 pagesCyclic Steam Stimulation Thermal EOR ProcessMohamed ElkumatiNo ratings yet
- ALCO Cat E 2009Document163 pagesALCO Cat E 2009SaMos AdRiianNo ratings yet
- Biology Internal Assessment 2Document9 pagesBiology Internal Assessment 2Hayley ChiuNo ratings yet
- Bulk Storage and Handling of Solvents and CoalescentsDocument8 pagesBulk Storage and Handling of Solvents and CoalescentslukmannyeoNo ratings yet
- Synthesis of Deuterated Dextromethorphan DerivativDocument13 pagesSynthesis of Deuterated Dextromethorphan DerivativCon Bò Sữa Thất TìnhNo ratings yet
- Title: Pre-Treatment of PC Blended FabricsDocument12 pagesTitle: Pre-Treatment of PC Blended FabricsFahad HussainNo ratings yet
- PUB Stipulated Standards Requirements For Water Fittings PDFDocument75 pagesPUB Stipulated Standards Requirements For Water Fittings PDFrajmohan198512No ratings yet
- Technical Delivery Condition For: Hot Rolled Carbon (Micro Alloy) SteelDocument2 pagesTechnical Delivery Condition For: Hot Rolled Carbon (Micro Alloy) SteelManjunath UNo ratings yet
- Laboratory Report CHM 213 (Physical Chemistry) : 1. Muhammad Mirza Hizami Bin RajieiDocument6 pagesLaboratory Report CHM 213 (Physical Chemistry) : 1. Muhammad Mirza Hizami Bin RajieiMuhd Mirza Hizami100% (2)
- Ekstraksi Dan Stabilitas Betasianin Daun Darah (Alternanthera Dentata) (KAJIAN PERBANDINGAN PELARUT Air:Etanol Dan Suhu Ekstraksi)Document9 pagesEkstraksi Dan Stabilitas Betasianin Daun Darah (Alternanthera Dentata) (KAJIAN PERBANDINGAN PELARUT Air:Etanol Dan Suhu Ekstraksi)Hendra PanggabeanNo ratings yet
- Anatomy & Physiology: Levels of Structural OrganizationDocument8 pagesAnatomy & Physiology: Levels of Structural OrganizationDOROLA CRIS JANELICA R.No ratings yet
- LAB - Testing Acids & BasesDocument3 pagesLAB - Testing Acids & BasesRita AnyanwuNo ratings yet
- Service Manual: CB 18S CB 27S CB 37SDocument85 pagesService Manual: CB 18S CB 27S CB 37SMack DieselNo ratings yet
- Colloids and Surfaces B: Biointerfaces: SciencedirectDocument9 pagesColloids and Surfaces B: Biointerfaces: SciencedirectFrederik RareNo ratings yet
- Root Canal DisinfectionDocument4 pagesRoot Canal DisinfectionKarizma TravelNo ratings yet