Professional Documents
Culture Documents
2 Thermal Conductivity of Metal Rod
Uploaded by
Mithilesh kumarOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
2 Thermal Conductivity of Metal Rod
Uploaded by
Mithilesh kumarCopyright:
Available Formats
Department of Mechanical Engineering
EXPERIMENT:
Title. Thermal Conductivity of a Metal Rod
Aim. To determine the thermal conductivity of a metallic rod.
Prior Knowledge: Phenomenon of heat conduction in metals, Fourier law
application to plane wall and hollow cylinders and variation of thermal
conductivity with temperature.
Practical Outcomes. After experiment performance students must be able to
PrO 1. Derivation of steady state heat conduction in plane wall and hollow
cylinders.
PrO 2. Variation of thermal conductivity at different section of metal rod
and heat loss in radial direction.
PrO 3. Nature of variation of thermal conductivity of conducting metal with
temperature.
APPARATUS:
Measuring flask, stopwatch, thermocouples with temperature indicators.
Introduction
Thermal conductivity is an important thermophysical property of
conducting materials, by the virtue of which the material conducts the heat
energy through it. From Fourier’s law of heat conduction, thermal
conductivity is defined as
Heat Transfer Lab Manual Mechanical Engineering 7
Department of Mechanical Engineering
Q dT dT
k = − = q
A dx dx
where Q= heat transfer rate, W
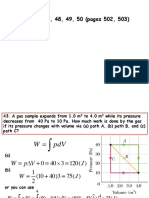
q= heat flux,W/m2
A= area normal to heat transfer,m2
dT
= temperature gradient in the direction of heat flow
dx
The thermal conductivity for a given material depends on its state, and it varies with 0the
direction, structure, humidity, pressure, and temperature change. Table 2.1shows the thermal
conductivity of some commonly used materials.
Thermal conductivities of some typical metals
Reference
Sr. No. Metals Thermal conductivity state
1 Aluminum 204 20°C
2 Pure copper 386 20°C
3 Brass 111 20°C
4 Pure iron 73 20°C
5 Silver 419 20°C
6 Stainless steel 16.2 20°C
2.3 MECHANISM OF HEAT CONDUCTION IN METALS
Thermal energy can be transported in solids by two means:
1. Lattice vibration
2. Transport of free electrons
Thermal energy can be conducted in solids by free electrons and by lattice vibrations. Large
numbers of free electrons move about in the lattice structure of the material in good
conductors. These electrons carry thermal energy from higher temperature region to lower
temperature region, in a similar way they transport electric charge. Infect, these electrons are
frequently referred as electron gas. The energy may also be transferred as vibrational energy
in the lattice structure of the material. However, this mode of energy transfer is not effective
as electron transport mechanism and hence, good electrical conductors are always good heat
conductors, e.g. copper, silver etc.
However, with increase in temperature, the lattice vibrations obstruct the transport of free
electrons in the material and therefore, for most the metals thermal conductivity decreases
with increase in temperature.
Heat Transfer Lab Manual Mechanical Engineering 8
Department of Mechanical Engineering
2.4 EXPERIMENTAL ANALYSIS
2.7.1 Experimental Setup
Fig.2.1 Experimental setup for determining the thermal conductivityofa metal bar
The experimental setup consists of a brass metallic bar as shown in Fig.2.1.The bar is
horizontally placed and its test section is surrounded by a thick layer of insulating material.
The bar is heated at one end with the help of a band-type electrical heater, and the other end
of the bar is projected into a cooling water jacket.
Six thermocouples T1 to T6 are embedded at known distances along the length of the bar in the
test section, and four thermocouples T7 to T10 are equipped in the insulating cylinder in order
to measure the heat loss in the radial direction. The inlet and outlet temperature of the
circulating water is measured by thermocouples T11 and T12, respectively. Fig.2.2 shows
Thermal Engineering Mechanical Engineering 9
Department of Mechanical Engineering
experimental arrangement in laboratory.
Fig.2.1 Experimental Setup
2.7.2 Theory
The bar is analyzed at two sections along its length (Figure 3). According to the first law of
thermodynamics, at any section the rate of incoming energy must be equal to the rate of
outgoing energy.
Rate of heat gain by circulating water = Rate of heat conduction at the free end of the rod
the rate of heat energy that reaches the circulating water is
Qw = m w C (T1 2 − T1 1 )
pw
…(1)
Where
mw= Mass flow rate of water in kg/s,
C pw
= Specific heat of water in J/kg.K
T12– T11= temperature difference of circulating water, °C
The rate of heat conduction through section B–B
2 L1 k in s ( T8 − T9 )
Q BB = Q + …(2)
W
r
In 0
ri
Where L1=spacing between section B-B and the cooling end
kins =conductivity of the insulating material packed around the test section
Thermal Engineering Mechanical Engineering 10
Department of Mechanical Engineering
ro= radius at which thermocouple 10is embedded
ri= radius at which thermocouple 9 is attached
T9= outer temperature of insulation at section B–B
T8 = inner temperature of insulation at section B–B
Further, the conduction rate at section A–A is expressed as
dT
Q = −k A (3)
BB B
dx BB
Where kB= Thermal conductivity of the bar at section B–B,
A= cross-sectional area of the bar, m2
dT
= temperature gradient at section B–B
dx BB
From Eqs. (2) and (3), we get
2 L (T − T )
QW + 1 8 9
r
In o
ri
kBB = …(4)
dT
A
dx B B
Similarly at section A–A we have
2 L 2 k in s ( T 6 − T 7 )
Q AA = Q BB + …(5)
r
In o
ri
Where L2= length of bar between section A-A and B-B
The thermal conductivity of the bar material at section A–A in terms of the heat
conduction rate is expressed as
2 L k (T − T )
Q BB +
2 ins 7 8
r
o
In
kAA= ri …(6)
dT
A
dx AA
Thermal Engineering Mechanical Engineering 11
Department of Mechanical Engineering
2.5 Procedure
1. Start the water supply through the water jacket and regulate its uniform flow rate.
2. Turn on the heater switch and adjust the heater input through the dimmer-stat.
3. Wait until the steady-state condition is reached.
4. Note the reading of all thermocouples through the selector switch, voltmeter,
and ammeter.
5. Plotthetemperatureagainstthelocationofthethermocouplesalongthetestsectionof the
bar.
2.6 Specifications
1. Total length of the bar = 505 mm
2. Test length of the bar L= 400mm
3. Diameter of the bar d = 25mm
4. Temperature of the bar = 0–300°C
5. Thermocouple type = ChromelAlumel
6. Measuring flask capacity = 0 to 1000ml
7. Spacing between thermocouples =mm
8. Specific heat of the water Cpw= 4187 J/kg·K
9. Thermal conductivity of insulating material kins= 0.15 W/m·K
10. Insulation along the test length of rod Glass wool.
2.7 Observation Table
Mass Heater
Thermocouple readings, °C
Sr. flow input
No. (kg/s) V I 1 2 3 4 5 6 7 8 9 10 11 12
Thermal Engineering Mechanical Engineering 12
Department of Mechanical Engineering
2.10 Calculation
Using the temperatures of the bar at various points, plot the temperature distribution T1 to T6
along the length of the bar and determine the slopes of the graph dT/dx at the sections AA,
BB, CC as shown in Fig. 2.3 below.
Fig. 2.3 Temperature distribution along the length of a metal bar
(Note: The value of temperature goes on decreasing along the length of the bar, and the
value of slope dT/dx is always negative.)
Heat is flowing through the bar from heater end to water heat sink. When steady state
is reached, heat passing through the section CC of the bar is equal to heat gain by water.
Use Eqs. (1) to (6) to calculate the result.
Thermal Engineering Mechanical Engineering 13
Department of Mechanical Engineering
CALCULATIONS FOR READING
Thermal Engineering Mechanical Engineering 14
Department of Mechanical Engineering
2.11RESULT
Take the average of the two values of thermal conductivity and use it as the outcome of
the experiment.
The average thermal conductivity of the brass = W/m·K
2.12. Conclusions
1. Compare the value of thermal conductivity obtained by experimentation with a
standard value. State the reason for any deviation.
2. From the experiment, it is concluded that the temperature goes on decreasing along the
length of the rod.
3. The thermal conductivity of brass decreases with the increase in temperature. This is
due to an obstruction in the flow of free electrons caused by the increase in the
amplitude of the lattice vibration.
2.13PRECAUTIONS
1. Wait until perfect steady state is reached.
2. Input supply must be constant.
3. Handle the change-over switch of temperature gently.
QUESTIONS:
1. Define thermal conductivity.
2. What is meant by “one dimensional steady state ‟ when applied to conduction problem.
3. Give order of thermal conductivity for copper, brass, stainless steel.
4. Give thermal conductivity of any five metals.
5. Identify the mode of heat transfer in the following
ANSWERS: Attached Separate sheet
Thermal Engineering Mechanical Engineering 15
Department of Mechanical Engineering
Thermal Engineering Mechanical Engineering 16
You might also like
- Solution Manual for an Introduction to Equilibrium ThermodynamicsFrom EverandSolution Manual for an Introduction to Equilibrium ThermodynamicsNo ratings yet
- Ex. No 1) Thermal Conductivity of Metal RodDocument8 pagesEx. No 1) Thermal Conductivity of Metal RodGaurav Sonawane0% (1)
- Heat Transfer by S K Mondal-3-42Document40 pagesHeat Transfer by S K Mondal-3-42Arpit Thakur0% (2)
- Heat Transfer IES GATE IAS 20 Years Question and Answers by S K MondalDocument115 pagesHeat Transfer IES GATE IAS 20 Years Question and Answers by S K Mondalraju100% (1)
- 10c.heat TransferDocument4 pages10c.heat TransferJatin SonwalNo ratings yet
- Advances in Magnetohydrodynamics: Proceedings of a Colloquium Organized by the Department of Fuel Technology and Chemical Engineering at Sheffield University, October 1961From EverandAdvances in Magnetohydrodynamics: Proceedings of a Colloquium Organized by the Department of Fuel Technology and Chemical Engineering at Sheffield University, October 1961I. A. McGrathNo ratings yet
- Heat Transfer PDFDocument40 pagesHeat Transfer PDFVivek SharmaNo ratings yet
- Write Up Thermal Conductivity of RodDocument10 pagesWrite Up Thermal Conductivity of RodRutuja DeshmukhNo ratings yet
- Conduction of Heat in Metal-RodDocument9 pagesConduction of Heat in Metal-RodOmkar HaranNo ratings yet
- SMT - Kashibai Navale College of Engineering, Vadgaon Pune: Heat TransferDocument8 pagesSMT - Kashibai Navale College of Engineering, Vadgaon Pune: Heat TransferFS18ME046 MAYUR NikamNo ratings yet
- Metal Rod-24-30Document7 pagesMetal Rod-24-30Vidhya NairNo ratings yet
- Alagappa Chettiar Government College of Engineering and TechnologyDocument86 pagesAlagappa Chettiar Government College of Engineering and TechnologyASWIN VICTOR .ENo ratings yet
- HMT Lab ManualDocument33 pagesHMT Lab ManualMahikant JhaNo ratings yet
- Kuliah 4 Dan 5 Konduksi SteadyDocument61 pagesKuliah 4 Dan 5 Konduksi Steadyandreasjoni92No ratings yet
- HMT DAVV PracticalDocument21 pagesHMT DAVV PracticalNarendraNo ratings yet
- HT Lab Manual - Cycle 2Document22 pagesHT Lab Manual - Cycle 2konda Sasi Snehith reddyNo ratings yet
- Gate Questions Bank ME HMTDocument4 pagesGate Questions Bank ME HMTTaanzNo ratings yet
- Thermal Conductivity of A Metal Rod: Aim & ObjectiveDocument8 pagesThermal Conductivity of A Metal Rod: Aim & ObjectivekishoreNo ratings yet
- Chapter 2 Heat ConductionDocument26 pagesChapter 2 Heat ConductionFarooq AhmadNo ratings yet
- Thermal Conductvity Matal RodDocument7 pagesThermal Conductvity Matal RodTheOPGarriXNo ratings yet
- Week 2 - Assignment 2 - Final Heat TransferDocument4 pagesWeek 2 - Assignment 2 - Final Heat TransferchandrakiranNo ratings yet
- Kom Manual PDFDocument44 pagesKom Manual PDFShivam DoharNo ratings yet
- Heat Transfer Exercise With AnswerKeyDocument13 pagesHeat Transfer Exercise With AnswerKeyRitesh BNo ratings yet
- Assignment02 KM32203Document3 pagesAssignment02 KM32203Raiyre RolandNo ratings yet
- AP Government GATE Online Classes: Day-1 (26.05.2020) Dr. R. Srikanth Professor ANITS-VisakhapatnamDocument64 pagesAP Government GATE Online Classes: Day-1 (26.05.2020) Dr. R. Srikanth Professor ANITS-VisakhapatnamConceptual GATE & ESENo ratings yet
- CH2 Heat Transfer 2Document19 pagesCH2 Heat Transfer 2abdoasdafm7No ratings yet
- Answer 2 16 Marks HMTDocument64 pagesAnswer 2 16 Marks HMTfahamith ahamed100% (1)
- Heat and Mass Transfer by S K Mondal T&QDocument216 pagesHeat and Mass Transfer by S K Mondal T&Qajaykrishna_9983% (6)
- "We Have A Tradition of Being Awesome.": Quadtech ChemicalsDocument9 pages"We Have A Tradition of Being Awesome.": Quadtech ChemicalsKamran AliNo ratings yet
- Heat TransferDocument14 pagesHeat Transferbackupamey2No ratings yet
- Da 5Document22 pagesDa 5Abdur RehmanNo ratings yet
- Experiment 3Document8 pagesExperiment 3ashutosh yagyikNo ratings yet
- Calculation of Thermal Contact ResistanceDocument3 pagesCalculation of Thermal Contact ResistanceAhmed QasimNo ratings yet
- Modes of Heat Transfer: O Q (Gate, Ies, Ias)Document7 pagesModes of Heat Transfer: O Q (Gate, Ies, Ias)ankitNo ratings yet
- LN13 PDFDocument54 pagesLN13 PDFelty TanNo ratings yet
- HEat Transfer DPP 46Document46 pagesHEat Transfer DPP 46Dev PancholiNo ratings yet
- Thermal Conductivity of Metal RodDocument5 pagesThermal Conductivity of Metal RodinfoNo ratings yet
- Chapterwise Super Thirty FinalDocument158 pagesChapterwise Super Thirty FinalAnil KumarNo ratings yet
- Lecture05 P2Document20 pagesLecture05 P2Brandy TranNo ratings yet
- Steady State of ConductionDocument6 pagesSteady State of ConductionLOPIGA, HERSHA MHELE A.No ratings yet
- ISA-1 - Solutions - HMT - 17 May 2021Document5 pagesISA-1 - Solutions - HMT - 17 May 2021Wild BotNo ratings yet
- H2: Radial Heat Conduction Experiment: 1. ObjectivesDocument5 pagesH2: Radial Heat Conduction Experiment: 1. ObjectivesOmar AhmedNo ratings yet
- Lesson 1-Heat Transfer, Importance of Heat Transfer, Modes of Heat TransferDocument3 pagesLesson 1-Heat Transfer, Importance of Heat Transfer, Modes of Heat Transfercharmaine fosNo ratings yet
- Chapter 13 PDFDocument42 pagesChapter 13 PDFevergarden33No ratings yet
- Assignment - Heat TransferDocument3 pagesAssignment - Heat TransferReyansh MittalNo ratings yet
- Exp 1 Heat ConductionDocument12 pagesExp 1 Heat ConductionDaniyal ZainurinNo ratings yet
- Assignment 1Document5 pagesAssignment 1Miriam NagyNo ratings yet
- Experiment No.: 1: Aim: Thermal Conductivity of Metal RodDocument4 pagesExperiment No.: 1: Aim: Thermal Conductivity of Metal RodHardik Kumar MendparaNo ratings yet
- Heat and Mass Transfer by S K Mondal T&Q (1) .0001Document110 pagesHeat and Mass Transfer by S K Mondal T&Q (1) .0001ksNo ratings yet
- Solution of Transient 2D Heat Conduction Problem Using Freefem++Document4 pagesSolution of Transient 2D Heat Conduction Problem Using Freefem++Chicca PantanoNo ratings yet
- Department of Chemical & Biomolecular Engineering The National University of SingaporeDocument6 pagesDepartment of Chemical & Biomolecular Engineering The National University of SingaporeHaha WoNgNo ratings yet
- FS-2 CPP 10 Physics Chemistry Mathematics 2020Document25 pagesFS-2 CPP 10 Physics Chemistry Mathematics 2020Durgesh SINGHNo ratings yet
- Physical Electronics: Handbook of Vacuum PhysicsFrom EverandPhysical Electronics: Handbook of Vacuum PhysicsA. H. BeckNo ratings yet
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)
- Thermal Engineering Lab ExperimentDocument1 pageThermal Engineering Lab ExperimentMithilesh kumarNo ratings yet
- 10 Assigments 02 - DMEDocument1 page10 Assigments 02 - DMEMithilesh kumarNo ratings yet
- 9 Assigments - 01 DmeDocument1 page9 Assigments - 01 DmeMithilesh kumarNo ratings yet
- 7 Lec DME Ergonomics of DesignDocument2 pages7 Lec DME Ergonomics of DesignMithilesh kumarNo ratings yet