Professional Documents
Culture Documents
Act 6 - Tabla de Atomos
Act 6 - Tabla de Atomos
Uploaded by
Ximena Lucia Grandez BreñaOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Act 6 - Tabla de Atomos
Act 6 - Tabla de Atomos
Uploaded by
Ximena Lucia Grandez BreñaCopyright:
Available Formats
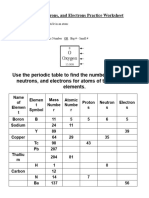
Part 2: Atom Table
In this activity you will need a Periodic Table and will need to apply these ‘rules’.
Atomic Number = number of protons (= number of electrons in a neutral atom)
Mass Number = number of protons + number of neutrons
Symbol for atom Atomic Mass Number Number Number
or ion Numbe Numbe of of of
r r protons electrons neutrons
Bi 83 126
Pt 195
Ra 88 138
Sn 50 119
Ag 108
27 13
O2- 8
12 10 13
Al3+ 14
17 37 18
Make up your own atoms/ions for the last two rows in the table
You might also like
- Semiconductor Data Book: Characteristics of approx. 10,000 Transistors, FETs, UJTs, Diodes, Rectifiers, Optical Semiconductors, Triacs and SCRsFrom EverandSemiconductor Data Book: Characteristics of approx. 10,000 Transistors, FETs, UJTs, Diodes, Rectifiers, Optical Semiconductors, Triacs and SCRsRating: 5 out of 5 stars5/5 (1)
- Worksheet: Atoms, Isotopes, and Ions AtomsDocument2 pagesWorksheet: Atoms, Isotopes, and Ions AtomsLeo Torres GarcíaNo ratings yet
- 2024 - Chapter3 - Atomic StructureDocument15 pages2024 - Chapter3 - Atomic Structurekaylamok3No ratings yet
- Problem Set 3 FA 2018Document2 pagesProblem Set 3 FA 2018Dancer Dhruv MakwanaNo ratings yet
- Lecture 7 Sub-Atomic RelationshipDocument5 pagesLecture 7 Sub-Atomic RelationshipkedeshiaNo ratings yet
- Chapter 02 ISM Chang 14eDocument7 pagesChapter 02 ISM Chang 14elsytb2000No ratings yet
- Annotated-Atomic Structure Bohr Models-1Document2 pagesAnnotated-Atomic Structure Bohr Models-1Ivania Joselina Lobo MontoyaNo ratings yet
- Solution Manual For Chemistry 11Th Edition by Chang Isbn 007766695X 9780077666958 Full Chapter PDFDocument36 pagesSolution Manual For Chemistry 11Th Edition by Chang Isbn 007766695X 9780077666958 Full Chapter PDFtiffany.kunst387100% (10)
- O-Level Atomic ST NotesDocument12 pagesO-Level Atomic ST Notesgangsterboy.since2023No ratings yet
- 3 Atoms and Elements: Core CurriculumDocument2 pages3 Atoms and Elements: Core CurriculumTagan TaganovNo ratings yet
- Atoms and The Periodic Table: OxygenDocument23 pagesAtoms and The Periodic Table: OxygenNatalia WhyteNo ratings yet
- Element Symbol: Atomic Number/ # ProtonDocument1 pageElement Symbol: Atomic Number/ # ProtonMJ SolNo ratings yet
- Topic2-Atoms, Molecules&Ions PpsDocument42 pagesTopic2-Atoms, Molecules&Ions PpsNur Farhana SuhaimiNo ratings yet
- Basic 1st 01 02Document8 pagesBasic 1st 01 02Taseem Ali KhanNo ratings yet
- Lilavatibai Podar High School (Isc) : Holding Capacity of Shells: 2 N Formula (N Position of The Shell From The NucleusDocument3 pagesLilavatibai Podar High School (Isc) : Holding Capacity of Shells: 2 N Formula (N Position of The Shell From The NucleusMahesh hamneNo ratings yet
- 2017 D 2.0 TCI-R D 2.0 TCI-R Schematic Diagrams Engine Electrical System Engine Control System Schematic DiagramsDocument1 page2017 D 2.0 TCI-R D 2.0 TCI-R Schematic Diagrams Engine Electrical System Engine Control System Schematic Diagramslyanna120168No ratings yet
- Protons Neutrons Electrons Review KEYDocument3 pagesProtons Neutrons Electrons Review KEYMiguel Jimenez OsorioNo ratings yet
- Solution Manual For Chemistry 11th Edition by Chang ISBN 007766695X 9780077666958Document36 pagesSolution Manual For Chemistry 11th Edition by Chang ISBN 007766695X 9780077666958henryarmstrongypajbizoqe100% (23)
- Dalton's Atomic Theory: Worksheet On General ChemistryDocument3 pagesDalton's Atomic Theory: Worksheet On General ChemistryMay Conde AguilarNo ratings yet
- Atoms and Atomic Structure ClassworkDocument5 pagesAtoms and Atomic Structure ClassworkJana OweiwiNo ratings yet
- By Vicki - The Science Lady: Atomic StructureDocument3 pagesBy Vicki - The Science Lady: Atomic StructureLeila BawabNo ratings yet
- CHM150 (Practice) (Atomic and Mass Number)Document1 pageCHM150 (Practice) (Atomic and Mass Number)sandraNo ratings yet
- Number of Protons WorksheetDocument4 pagesNumber of Protons WorksheetIrene SanchezNo ratings yet
- Grade 10 Chemistry Week 3 Lesson 2 Worksheet 2 and SolutionsDocument7 pagesGrade 10 Chemistry Week 3 Lesson 2 Worksheet 2 and SolutionsNikoli MajorNo ratings yet
- 1.chem Review & Aquesous Solutions Key.Document34 pages1.chem Review & Aquesous Solutions Key.Calo Is TrashNo ratings yet
- Atomic Structure & The Periodic Table 1 QPDocument8 pagesAtomic Structure & The Periodic Table 1 QPAisha Jakhro100% (1)
- AssignmentDocument6 pagesAssignmentSashant kapoorNo ratings yet
- Subatomic Particles - FILLDocument2 pagesSubatomic Particles - FILLALMERA SHELLA CABOGONo ratings yet
- Atomic Structure-OL-NotesDocument4 pagesAtomic Structure-OL-Notesshlaibat13No ratings yet
- Kelapa GadingDocument2 pagesKelapa GadingUtari Ika CahyaniNo ratings yet
- CH 2Document14 pagesCH 2dwarriorsNo ratings yet
- Group Activity1Document1 pageGroup Activity1ansalerochNo ratings yet
- Year 8 Atomic Structure & The Periodic Table 1 QPDocument3 pagesYear 8 Atomic Structure & The Periodic Table 1 QPjNo ratings yet
- Standard:: ANSWER KEYDocument23 pagesStandard:: ANSWER KEYYassue OfficialNo ratings yet
- All About The Periodic Table - Home Laboratory WorksheetDocument4 pagesAll About The Periodic Table - Home Laboratory WorksheetFrank Ed SerranoNo ratings yet
- Energía de AfinidadDocument3 pagesEnergía de AfinidadLINA MARCELA DELGADO CRUZNo ratings yet
- Chap 1 - Elementary Mat Science Concepts Rev 1Document50 pagesChap 1 - Elementary Mat Science Concepts Rev 1Jay MashNo ratings yet
- Apostila de An 2S11Document56 pagesApostila de An 2S11yebaho2182No ratings yet
- Atomic Structure: Activity 3Document6 pagesAtomic Structure: Activity 3Aanstein YalungNo ratings yet
- Atomic Structure 1Document27 pagesAtomic Structure 1Mamdooh AlqathamiNo ratings yet
- Worksheet 2 - Electrons Protons and NeutronsDocument3 pagesWorksheet 2 - Electrons Protons and NeutronsAnonymous ANo ratings yet
- Proton and Nucleon Number QDocument16 pagesProton and Nucleon Number QPuja DhawanNo ratings yet
- Proton and Nucleon Number QDocument16 pagesProton and Nucleon Number Qm6vs24rvwmNo ratings yet
- Graphing Periodic Trends LabDocument3 pagesGraphing Periodic Trends LabAbhi JainNo ratings yet
- HTPC Project 2Document26 pagesHTPC Project 2JephinSJohnNo ratings yet
- SDFGHJHFGDocument5 pagesSDFGHJHFGNafiul BariNo ratings yet
- General Chemistry Seatwork No. 14Document3 pagesGeneral Chemistry Seatwork No. 14BRYAN AVILESNo ratings yet
- Lecture (13) Residence Time Distribution and Reactor PerformanceDocument26 pagesLecture (13) Residence Time Distribution and Reactor Performanceمروان ابراهيم حمد عبدNo ratings yet
- Wk3 Atoms Ions Isotopes SPR23Document1 pageWk3 Atoms Ions Isotopes SPR23CardinylNo ratings yet
- Atom and Atomic StructureDocument8 pagesAtom and Atomic StructureAlex noslenNo ratings yet
- Chem Form-6-Sem-1-01 PDFDocument44 pagesChem Form-6-Sem-1-01 PDFXuerong ChinNo ratings yet
- Topic 1: Structure of An AtomDocument18 pagesTopic 1: Structure of An AtomElsw FewNo ratings yet
- 1 Grade 11 Review AnswersDocument9 pages1 Grade 11 Review Answersapi-363234558No ratings yet
- 1.1 Atoms and Molecules PDFDocument39 pages1.1 Atoms and Molecules PDFKKNo ratings yet
- KCSE Form 2 NotesDocument139 pagesKCSE Form 2 NotesN KatanaNo ratings yet
- Atomic Structure PacketDocument13 pagesAtomic Structure PacketPriti JainNo ratings yet
- Chapter (2) Atomic Structure and BondingDocument56 pagesChapter (2) Atomic Structure and BondingJessica De GuzmanNo ratings yet
- A Level Group 3 - 13 Boron Aluminium Chemistry P-Block Elements of The Periodic Table GCE AS A2 Inorganic Revision Notes KS5 PDFDocument11 pagesA Level Group 3 - 13 Boron Aluminium Chemistry P-Block Elements of The Periodic Table GCE AS A2 Inorganic Revision Notes KS5 PDFAlbertJnBaptisteNo ratings yet
- Bond TypesDocument28 pagesBond Typesvigneshs.me23No ratings yet
- Chapter 3 - SeatworkDocument2 pagesChapter 3 - SeatworkVincent DayangcoNo ratings yet
- Imagen MosquitoesDocument7 pagesImagen MosquitoesXimena Lucia Grandez BreñaNo ratings yet
- Instrucciones Animal Cell Vs Plant CellDocument1 pageInstrucciones Animal Cell Vs Plant CellXimena Lucia Grandez BreñaNo ratings yet
- Rubric Oral PresentationDocument4 pagesRubric Oral PresentationXimena Lucia Grandez BreñaNo ratings yet
- No Paine No GainDocument2 pagesNo Paine No GainXimena Lucia Grandez BreñaNo ratings yet
- WS Everyday Mystery - ENGDocument1 pageWS Everyday Mystery - ENGXimena Lucia Grandez BreñaNo ratings yet