0% found this document useful (0 votes)
3K views20 pagesFactors Affecting Cell Internal Resistance
1) The document is a physics project report submitted by a student named Tapan Boruah studying in class 12.
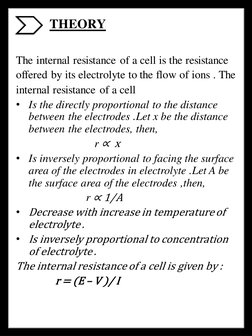
2) The project aims to study the various factors that affect the internal resistance of a cell through practical analysis, including the distance between electrodes, electrode area, electrolyte temperature, and electrolyte concentration.
3) The results show that the internal resistance of a cell is directly proportional to the distance between electrodes and inversely proportional to electrode area, electrolyte temperature, and electrolyte concentration.
Uploaded by
RΛJIBUL ISLΛMCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
3K views20 pagesFactors Affecting Cell Internal Resistance
1) The document is a physics project report submitted by a student named Tapan Boruah studying in class 12.
2) The project aims to study the various factors that affect the internal resistance of a cell through practical analysis, including the distance between electrodes, electrode area, electrolyte temperature, and electrolyte concentration.
3) The results show that the internal resistance of a cell is directly proportional to the distance between electrodes and inversely proportional to electrode area, electrolyte temperature, and electrolyte concentration.
Uploaded by
RΛJIBUL ISLΛMCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
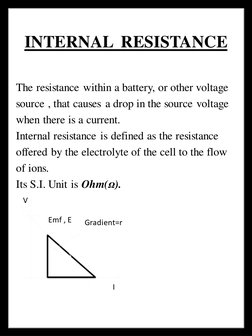
- Introduction: Introduces the need for studying battery internal resistance and sets context for the project.
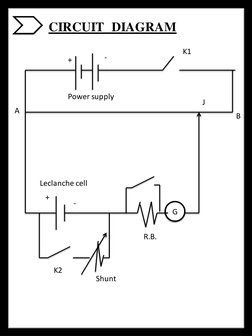
- Practical Analysis: Provides detailed analysis of the internal resistance in cells, including objectives, apparatus, theoretical background, circuit setup, procedures, observations, results, and possible errors.
- Conclusion: Summarizes the key factors affecting internal cell resistance using a flowchart.
- Bibliography: Lists resources and references used throughout the project documentation.