Professional Documents
Culture Documents
Atomic Theory - Checklist
Atomic Theory - Checklist
Uploaded by
Mary GinetaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Atomic Theory - Checklist
Atomic Theory - Checklist
Uploaded by
Mary GinetaCopyright:
Available Formats
ATOMIC THEORY
The Nuclear Atom
Essential idea: The mass of an atom is concentrated in its minute, positively charged nucleus.
ASSESSMENT OBJECTIVES D R R R
State the position of protons, neutrons and electrons in the atom.
State the relative masses and relative charges of protons, neutrons and
electrons.
* The accepted values are:
proton neutron electron
relative mass 1 1 negligible
relative charge + 0 -
Define the terms mass number (A), atomic number (Z) and isotopes of an
element.
Deduce the symbol for an isotope given its mass number and atomic number.
A
*The following notation are to be used: Z X
Calculate the number of protons, neutrons and electrons in atoms and ions from
the mass number, atomic number and charge.
Compare the properties of the isotopes of an element.
Discuss the uses of radioisotopes
*Examples include 14❑C
in radiocarbon dating, 60
❑Co
in radiotherapy, and 131
❑I
and 125
❑I
as medical tracers.
Calculate non-integer relative atomic masses and abundance of isotopes from
given data.
Electronic Configuration
Essential idea: The electron configuration of an atom can be deduced from its atomic number.
ASSESSMENT OBJECTIVES D R R R
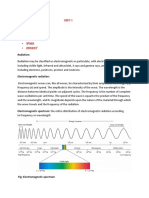
Describe the relationship between colour, wavelength, frequency and energy
across the electromagnetic spectrum.
Distinguish between a continuous spectrum and a line spectrum.
Describe the emission spectrum of the hydrogen atom, including the
relationships between the lines and energy transitions to the first, second and
third energy levels.
Recognize the shape of an s atomic orbital and the p x, py and pz atomic
orbitals.
The line emission spectrum of hydrogen provides evidence for the existence of
electrons in discrete energy levels, which converge at higher energies.
Identify the maximum number of electrons in the main energy level or shell is
given an integer number, n as 2 n2.
Describe the model of the atom in terms of the main energy level into s, p, d
and f sub-levels of successively higher energies.
Define orbitals as regions of space where there is a high probability of finding
an electron.
Apply the Aufbau principle, Hund’s rule and the Pauli exclusion principle to
write electron configurations for atoms and ions up to Z = 36.
* full electron configuration, condensed electron configurations , orbital diagrams
*The electron configurations of Cr and Cu as exceptions should be recognized.
You might also like
- Fundamentals of Energy Dispersive X-Ray Analysis: Butterworths Monographs in MaterialsFrom EverandFundamentals of Energy Dispersive X-Ray Analysis: Butterworths Monographs in MaterialsRating: 5 out of 5 stars5/5 (1)
- Hydrology and Water Management in The Humid Tropics - Hydrological Research Issues and Strategies For Water Management (International Hydrology Series) (PDFDrive) PDFDocument595 pagesHydrology and Water Management in The Humid Tropics - Hydrological Research Issues and Strategies For Water Management (International Hydrology Series) (PDFDrive) PDFRomie ZapantaNo ratings yet
- 1.1 Atomic StructureDocument21 pages1.1 Atomic StructureBhPO2023No ratings yet
- Basic Electrostatics (2019)Document21 pagesBasic Electrostatics (2019)Andile MinenhleNo ratings yet
- Capítulo 3Document13 pagesCapítulo 3José Daniel Campos MéndezNo ratings yet
- NM Physics (1st Lecture) - 1Document32 pagesNM Physics (1st Lecture) - 1FahadNo ratings yet
- Topic 2 - 12Document38 pagesTopic 2 - 12Iyvd sdfshethNo ratings yet
- AtomsDocument3 pagesAtomsTanya NdlovuNo ratings yet
- Chapter 4 - Structure of The AtomDocument59 pagesChapter 4 - Structure of The AtomIsaac LibuNo ratings yet
- Chemical Physics Letters: Hugo J. Bohórquez, Russell J. BoydDocument5 pagesChemical Physics Letters: Hugo J. Bohórquez, Russell J. BoydRudolf KiraljNo ratings yet
- Igcse Chemistry Study Notes Unit 3 Atoms, Elements & CompoundsDocument12 pagesIgcse Chemistry Study Notes Unit 3 Atoms, Elements & CompoundsholaNo ratings yet
- 1.nuclear PharmacyDocument40 pages1.nuclear PharmacySheetal MeeniaNo ratings yet
- Basic ConceptsDocument39 pagesBasic Conceptsrejie magnayeNo ratings yet
- Atoms and NucleiDocument12 pagesAtoms and NucleiBablu ChaudharyNo ratings yet
- Unit 9Document26 pagesUnit 9Amol ShindeNo ratings yet
- STD 8 Chapter 5Document6 pagesSTD 8 Chapter 5ROHIT KADAMNo ratings yet
- Radiation PhysicsDocument26 pagesRadiation Physicsnirmalya prasun nayakNo ratings yet
- Nuclear StructureDocument37 pagesNuclear StructureNaresh KumarNo ratings yet
- Atomic Structure and Atomic Mass - NCUKDocument27 pagesAtomic Structure and Atomic Mass - NCUKphonepyaehtut2006No ratings yet
- Atomic Models - TheoryDocument109 pagesAtomic Models - TheoryDr-Walid FemtosecondNo ratings yet
- Atomic StructureDocument57 pagesAtomic StructuremichalinaNo ratings yet
- Ib Chemistry: Higher LevelDocument72 pagesIb Chemistry: Higher LeveldeveenNo ratings yet
- NCERT PUNCH Chemistry Class 11 Complete Book Flattened SignedDocument304 pagesNCERT PUNCH Chemistry Class 11 Complete Book Flattened Signedsd0806787100% (1)
- H2 Chemistry (9729) Lecture Notes 3 Atomic Structure: Assessment ObjectivesDocument30 pagesH2 Chemistry (9729) Lecture Notes 3 Atomic Structure: Assessment ObjectivesArvin LiangdyNo ratings yet
- Structure of AtomDocument27 pagesStructure of AtomGautam AryaNo ratings yet
- Structure of AtomDocument6 pagesStructure of AtomAbhishekkpNo ratings yet
- 1.1 Atoms and MoleculesDocument60 pages1.1 Atoms and MoleculesMOHAMAD FIRDAUS BIN HARUN KM-PensyarahNo ratings yet
- Dasar-Dasar Radiofarmasi: Lecturer: Prof. Resmi Mustarichie MSC, PH.D, AptDocument25 pagesDasar-Dasar Radiofarmasi: Lecturer: Prof. Resmi Mustarichie MSC, PH.D, Aptkiki ikrimaNo ratings yet
- 2 Atomic StructureDocument3 pages2 Atomic StructureJNo ratings yet
- Atomic Structure and The Periodic TableDocument15 pagesAtomic Structure and The Periodic TableBara' HammadehNo ratings yet
- Module Nuc - Phys I (Phys382) New1Document26 pagesModule Nuc - Phys I (Phys382) New1davididosa40No ratings yet
- 3 - Atomic Structure - History of AtomDocument15 pages3 - Atomic Structure - History of AtomVimanan A/L S. VelangganiNo ratings yet
- 11 It The Production, Properties and Interactions of X-RaysDocument14 pages11 It The Production, Properties and Interactions of X-Raysroma ulinaNo ratings yet
- 02-MS-ME - Atomic Structure and BondingDocument70 pages02-MS-ME - Atomic Structure and Bondingfarah Al-zgoulNo ratings yet
- Chapter 6 NewDocument59 pagesChapter 6 NewhoiminhNo ratings yet
- 1.1 Atoms and MoleculesDocument54 pages1.1 Atoms and MoleculesAbdullah AhmadNo ratings yet
- Lesson 20-10-2020-Nuclear ChemistryDocument10 pagesLesson 20-10-2020-Nuclear ChemistryAlemkeng BrendaNo ratings yet
- Atomic StructureDocument5 pagesAtomic StructureSiya ChiniahNo ratings yet
- Unit-1 Learning Objective Radiation Units Work EnergyDocument31 pagesUnit-1 Learning Objective Radiation Units Work EnergyManisha khanNo ratings yet
- Basic Chemistry NotesDocument81 pagesBasic Chemistry NotesRushikesh Navnath VarpeNo ratings yet
- Science FundamentalsDocument19 pagesScience FundamentalsSpa DNo ratings yet
- Prepared By: Aengel F. SamoranosDocument23 pagesPrepared By: Aengel F. SamoranosMikylla LimNo ratings yet
- PHS A DSE B1 TH Lecture 1Document12 pagesPHS A DSE B1 TH Lecture 1inzy283No ratings yet
- Nuclear Chemistry LectureDocument12 pagesNuclear Chemistry LectureAMLU Law OfficesNo ratings yet
- Topic:: STPM Term 1 ChemistryDocument47 pagesTopic:: STPM Term 1 ChemistryMenaga A/P IlangkovanNo ratings yet
- Structure of AtomDocument10 pagesStructure of Atomamogh sridharNo ratings yet
- Atomic Structure - Study NotesDocument16 pagesAtomic Structure - Study NotesTamoghna DeyNo ratings yet
- Atomic SructureDocument6 pagesAtomic SructureFrancis EssilfieNo ratings yet
- Module 2 (Atomic Structure and Interatomic Bonding)Document26 pagesModule 2 (Atomic Structure and Interatomic Bonding)Ralph Andrew Silverio100% (4)
- 2-Revision Atomic Structure & Periodic TableDocument14 pages2-Revision Atomic Structure & Periodic TableMohamudNo ratings yet
- Asc0304 Chemistry 1 Chapter 2: Atomic StructureDocument17 pagesAsc0304 Chemistry 1 Chapter 2: Atomic StructurehadassahhadidNo ratings yet
- Ib Atomic StructureDocument24 pagesIb Atomic Structurezarna nirmal rawalNo ratings yet
- Nuclear Engineering NotesDocument36 pagesNuclear Engineering NotesMatthew CurmiNo ratings yet
- Inside The Atom: Particle Physics Lesson 1Document19 pagesInside The Atom: Particle Physics Lesson 1Avinash BoodhooNo ratings yet
- Atomic StructureDocument21 pagesAtomic Structureamaya chopraNo ratings yet
- STPM Sem 1 Introductory Class Notes 2020Document12 pagesSTPM Sem 1 Introductory Class Notes 2020Johnson116No ratings yet
- CBSE Class 12 Physics Atoms & Nuclei Chapter Notes and Important Questions - 0 PDFDocument7 pagesCBSE Class 12 Physics Atoms & Nuclei Chapter Notes and Important Questions - 0 PDFNirmalNo ratings yet
- Unit 2+12 TARGET SHEET HL Atomic StructureDocument2 pagesUnit 2+12 TARGET SHEET HL Atomic Structurediya joshiNo ratings yet
- CHP 1 Basic Principle of ElectricityDocument22 pagesCHP 1 Basic Principle of ElectricityHasnain TanveerNo ratings yet
- Study Guide 5-Science 119Document8 pagesStudy Guide 5-Science 119bryanNo ratings yet
- Concepts of Nuclear Medicine Volume I: Concepts of Nuclear Medicine, #1From EverandConcepts of Nuclear Medicine Volume I: Concepts of Nuclear Medicine, #1No ratings yet
- Wagner Worksheet1Document6 pagesWagner Worksheet1Mary GinetaNo ratings yet
- UT1 SUVAT ChecklistDocument1 pageUT1 SUVAT ChecklistMary GinetaNo ratings yet
- Measurement Data AnalysisDocument13 pagesMeasurement Data AnalysisMary GinetaNo ratings yet
- Organic Chem Packet - SLDocument10 pagesOrganic Chem Packet - SLMary GinetaNo ratings yet
- Antibiotics JournalDocument13 pagesAntibiotics JournalMary GinetaNo ratings yet
- SucroseDocument4 pagesSucroseMary GinetaNo ratings yet
- AcidityDocument20 pagesAcidityMary GinetaNo ratings yet
- J-Garlic in CheeseDocument12 pagesJ-Garlic in CheeseMary GinetaNo ratings yet
- Golf StudyDocument25 pagesGolf StudyMary GinetaNo ratings yet
- J-Yogurt FactorsDocument9 pagesJ-Yogurt FactorsMary GinetaNo ratings yet
- GlucoseDocument10 pagesGlucoseMary GinetaNo ratings yet
- J - Pretreatment Methods - GerminationDocument6 pagesJ - Pretreatment Methods - GerminationMary GinetaNo ratings yet
- J-Natural Plant Enzyme InhibitorsDocument10 pagesJ-Natural Plant Enzyme InhibitorsMary GinetaNo ratings yet
- J-Bromelain EnzymeDocument9 pagesJ-Bromelain EnzymeMary GinetaNo ratings yet
- J - Pretreatment GerminationDocument9 pagesJ - Pretreatment GerminationMary GinetaNo ratings yet
- Kaori EnglishDocument15 pagesKaori EnglishMuna AjapNo ratings yet
- Green Building Materials PDFDocument6 pagesGreen Building Materials PDFdhanya1995No ratings yet
- 4RevisedECONOMIC TRAITS of Layers and BroilersDocument27 pages4RevisedECONOMIC TRAITS of Layers and BroilersRamesh BeniwalNo ratings yet
- Marketing - Module 8 The Marketing Mix - PLACEDocument10 pagesMarketing - Module 8 The Marketing Mix - PLACEKJ JonesNo ratings yet
- Clavicle FractureDocument15 pagesClavicle Fracturesalsabil aurellNo ratings yet
- CHN Pnle Board Exam Practice-Part 2Document6 pagesCHN Pnle Board Exam Practice-Part 2jerarddaria.elakNo ratings yet
- Sds Drive DC-PWM Compatible: EnglishDocument46 pagesSds Drive DC-PWM Compatible: EnglishderbalijalelNo ratings yet
- Smpep - Solar SusieDocument94 pagesSmpep - Solar SusieRavindraNo ratings yet
- PrematurityDocument37 pagesPrematurityNishaThakuriNo ratings yet
- MarraigeDocument5 pagesMarraigeshubhrataNo ratings yet
- Journal of Life Sciences & BiomedicineDocument8 pagesJournal of Life Sciences & BiomedicineBiomedicince journalNo ratings yet
- Hill's Healthy Weight Protocol Morphometric Measurement Instructions - FELINEDocument1 pageHill's Healthy Weight Protocol Morphometric Measurement Instructions - FELINEDakochan 26No ratings yet
- Jonsered Chain Guide 72LGX - F&BDocument2 pagesJonsered Chain Guide 72LGX - F&BMycastNo ratings yet
- Generalized Anxiety Disorder (Gad)Document4 pagesGeneralized Anxiety Disorder (Gad)docshNo ratings yet
- Bayard K1 21Document11 pagesBayard K1 21ddrddrNo ratings yet
- Chestnut's Obstetric Anesthesia Principles and Practice, Fifth EditionDocument2 pagesChestnut's Obstetric Anesthesia Principles and Practice, Fifth EditionYohanes TjandraNo ratings yet
- Dairy Science and TechnologyDocument83 pagesDairy Science and Technologyیاسمین ثریاNo ratings yet
- EvaporationDocument7 pagesEvaporationPoonatiGiriNo ratings yet
- Transgender Cadet PolicyDocument8 pagesTransgender Cadet PolicyCadet Library100% (1)
- ALDRUM Drum ThickenerDocument2 pagesALDRUM Drum ThickenerPopo GisbertNo ratings yet
- ALCHEMY The Philosphers Stone by Alchemist NDCDocument3 pagesALCHEMY The Philosphers Stone by Alchemist NDCVladimir VergunNo ratings yet
- NCP For BreathingDocument17 pagesNCP For BreathingCeleste Sin Yee100% (1)
- Jurnal SklerosisDocument7 pagesJurnal SklerosisReginNo ratings yet
- Antimicrobial Activity of Essential Oil Against Oral StrainDocument6 pagesAntimicrobial Activity of Essential Oil Against Oral StrainIzabela IvanNo ratings yet
- Catchpole-2015 EG Morococha-Fluids PDFDocument33 pagesCatchpole-2015 EG Morococha-Fluids PDFMiguel Angel Catunta ZarateNo ratings yet
- Template Reimburse Dinas Luar KotaDocument2 pagesTemplate Reimburse Dinas Luar KotapnharyonoNo ratings yet
- Accident and Loss StatisticsDocument38 pagesAccident and Loss StatisticsAsim FarooqNo ratings yet
- Note-Taking From ReadingDocument7 pagesNote-Taking From ReadingJazelle Tenuki Rehara GunarathneNo ratings yet
- Institute of Mathematics: Linear Programming AssignmentsDocument4 pagesInstitute of Mathematics: Linear Programming AssignmentsRayyan CablesNo ratings yet