Professional Documents
Culture Documents
Oxidation Number
Uploaded by
Oliver Pereyra KnizeOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Oxidation Number
Uploaded by
Oliver Pereyra KnizeCopyright:
Available Formats
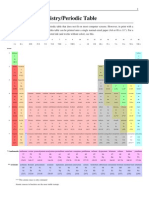
Physical Science Name: __________________________ Block: __
Oxidation Number Chart
Complete the following chart. If the element does not typically form ions, leave the last two
columns blank for it.
Number of Number of Number of Type of Ion
Atomic Oxidation
Element Protons Electrons Valence Formed
Number Number
(+) (-) Electrons (+ or -)
1 1
Hydrogen 1 1 + 1-
Helium 2 2 2 2 + 0
3
Lithium 3 3 1 + 1+
Beryllium 4 4 4 2 + 2+
Boron 5 5 5 3 + 3+
6 4-/+
Carbon 6 6 4 -/+
Nitrogen 7 7 7 5 - 3-
Oxygen 8 8 8 6 - 2-
Fluorine 9 9 9 7 - 1-
Neon 10 10 10 8 + 0
Sodium 11 11 11 1 + 1+
+
Magnesium 12 12 12 2 2+
3+
Aluminum 13 13 13 3 +
Silicon 14 14 14 4 - +/- 4
15
Phosphorus 15 15 5 - 3-
6
Sulfur 16 16 16 - 2-
17
Chlorine 17 17 7 - 1-
Argon 18 18 18 8 + 0
Potassium
19 19 19 1 + 1+
2
Calcium 20 20 20 + 2+
psws0204.doc
You might also like
- 1 Atomic Structure and Ions AnswersDocument2 pages1 Atomic Structure and Ions AnswersAwais NaeemNo ratings yet
- Periodic Table WorksheetDocument2 pagesPeriodic Table WorksheetMaria Palavecino100% (1)
- Periodic Table of ElementsDocument1 pagePeriodic Table of ElementsaaminahcNo ratings yet
- Indiabix ThermodynamicsDocument41 pagesIndiabix Thermodynamicsjuswa pirizNo ratings yet
- Optical Engineering FundamentalsDocument292 pagesOptical Engineering Fundamentalseduardo0% (1)
- Solid State PhysicsDocument417 pagesSolid State Physicsapi-377220450% (2)
- Physical and Chemical Changes (Worksheet) : Name: Surname: School: Q.1. Define The Following Terms: ADocument2 pagesPhysical and Chemical Changes (Worksheet) : Name: Surname: School: Q.1. Define The Following Terms: AAngelique Pabillona90% (10)
- Eim-11 q1 w1 Mod1.PDF-editedDocument11 pagesEim-11 q1 w1 Mod1.PDF-editedMarie TuraNo ratings yet
- Periodic Table With IonsDocument1 pagePeriodic Table With IonstwmittlerNo ratings yet
- Jadual BerkalaDocument2 pagesJadual BerkalaRohani Yusof75% (4)
- PrincesDeGuia - PhET Simulation - Build An AtomDocument4 pagesPrincesDeGuia - PhET Simulation - Build An AtomRosana BercadesNo ratings yet
- Chemistry Shit Page 1Document1 pageChemistry Shit Page 1Ashfaq UddinNo ratings yet
- Tabela IonsDocument1 pageTabela IonsMatheus EduardoNo ratings yet
- Atomic Particle Practice - Subatomic Particles Worksheet (Fill in The Missing Information) Element - StudocuDocument1 pageAtomic Particle Practice - Subatomic Particles Worksheet (Fill in The Missing Information) Element - Studocuyohanorozco22No ratings yet
- Periodic Table PDFDocument1 pagePeriodic Table PDFaaliyahNo ratings yet
- 0 Solid Liquid Gas Synthetic: The Periodic Table of ElementsDocument1 page0 Solid Liquid Gas Synthetic: The Periodic Table of ElementsDragos AndrianaNo ratings yet
- Standard Reduction PotentialsDocument1 pageStandard Reduction PotentialsCamiloNo ratings yet
- Lecture 7 - Stability DiagramsDocument7 pagesLecture 7 - Stability DiagramsAththur MaulanaNo ratings yet
- Periodic Table of The Elements: Non-MetalsDocument1 pagePeriodic Table of The Elements: Non-MetalsAshley SmearerNo ratings yet
- Theoretical Preliminary Test ResultsDocument5 pagesTheoretical Preliminary Test ResultsThea Viktoria OgalescoNo ratings yet
- Mokeur Periodic TablecolDocument1 pageMokeur Periodic TablecolDaniel Jay KutzikNo ratings yet
- Development of Baking Powder: Landmark Lesson PlanDocument17 pagesDevelopment of Baking Powder: Landmark Lesson PlanAtom NerdNo ratings yet
- Periodic Table of The ElementsDocument1 pagePeriodic Table of The Elementskaren listNo ratings yet
- Class00 Science G10 Periodic TableDocument2 pagesClass00 Science G10 Periodic TableDora DuanNo ratings yet
- Redox ReactionsDocument1 pageRedox Reactionsthimansayeshini00No ratings yet
- Handout PolyatomicsDocument2 pagesHandout PolyatomicsBrandon2017No ratings yet
- Chem Memorizing TableDocument2 pagesChem Memorizing TableBill WongNo ratings yet
- Periodic Table of Elements W Oxidation States PubChemDocument1 pagePeriodic Table of Elements W Oxidation States PubChemSHENIVEL BANTENo ratings yet
- Periodic TableDocument1 pagePeriodic TablechiちNo ratings yet
- 7.1 Atomic Number and Mass NumberDocument3 pages7.1 Atomic Number and Mass NumberMuzammil HassanNo ratings yet
- 9PS Ion Notation WorksheetDocument4 pages9PS Ion Notation WorksheetKaden AguiarNo ratings yet
- First 20 Elements 2022 - Sheet1Document1 pageFirst 20 Elements 2022 - Sheet1shintaro midorimaNo ratings yet
- Chapter 4 - StoihiometryDocument12 pagesChapter 4 - StoihiometrySyahla Aurelya Djailani 7ANo ratings yet
- Elements Pics Simple 11x8.5 PDFDocument1 pageElements Pics Simple 11x8.5 PDFIsaac GarciaNo ratings yet
- Periodic Elements and Ionic ChargesDocument2 pagesPeriodic Elements and Ionic ChargeskjfhghjfggjfNo ratings yet
- Elements Pics Simple 11x8.5Document1 pageElements Pics Simple 11x8.5slunavaNo ratings yet
- Elements Pics Simple 11x8.5 PDFDocument1 pageElements Pics Simple 11x8.5 PDFWilde RilkeNo ratings yet
- Acs Periodic Table Poster - Download PDFDocument1 pageAcs Periodic Table Poster - Download PDFDhara PandeyNo ratings yet
- Gráficas GeochemDocument5 pagesGráficas Geochemvandrake10No ratings yet
- Periodic Table of Elements - With Added InfoDocument1 pagePeriodic Table of Elements - With Added InfoEdgar Clyde LopezNo ratings yet
- Table of IonsDocument2 pagesTable of IonsLucia Jimenez AlvarezNo ratings yet
- IUPAC Periodic Table-22Jun07bDocument1 pageIUPAC Periodic Table-22Jun07bAdnan Ali100% (2)
- Sci 10 Data BookletDocument7 pagesSci 10 Data BookletConstanza Vitulli RoqueNo ratings yet
- Water ChemDocument4 pagesWater ChemZahurul IslamNo ratings yet
- Atomic MassDocument1 pageAtomic MassDeepti JainNo ratings yet
- Elements and The Periodic Table WorksheetDocument4 pagesElements and The Periodic Table WorksheetVictoria StewartsonNo ratings yet
- The Periodic Table of Elements: Daniel LundbergDocument2 pagesThe Periodic Table of Elements: Daniel LundbergEZLYEN AZLINNo ratings yet
- Tabla Periodica RosmeryDocument4 pagesTabla Periodica Rosmeryfeilx3donutNo ratings yet
- The Periodic Table of Elements: Daniel LundbergDocument2 pagesThe Periodic Table of Elements: Daniel LundbergOgunbowale Olatayo BodunrinNo ratings yet
- Periodic TableDocument1 pagePeriodic TableAutumn EverettNo ratings yet
- The Periodic Table of Elements 2018 ColorDocument2 pagesThe Periodic Table of Elements 2018 ColorAayush GuptaNo ratings yet
- The Periodic Table of Elements: Daniel LundbergDocument2 pagesThe Periodic Table of Elements: Daniel LundbergAHNAF AJMAINNo ratings yet
- Periodic Table Color 2017Document1 pagePeriodic Table Color 2017yahooincNo ratings yet
- ChemistryDocument3 pagesChemistryMurphy_AMDNo ratings yet
- Ions & Isotopes PracticeDocument1 pageIons & Isotopes PracticeSamAndPuffNo ratings yet
- Periodic Table of The Elements: Appendix CDocument2 pagesPeriodic Table of The Elements: Appendix CMichelle NgNo ratings yet
- The Periodic Table of Elements 2018 ColorDocument2 pagesThe Periodic Table of Elements 2018 ColorCawf KgfNo ratings yet
- Ion Worksheet KEYDocument1 pageIon Worksheet KEYAna Marie Corales TabunarNo ratings yet
- B & W Periodic TableDocument1 pageB & W Periodic Tableshubham dagaleNo ratings yet
- da3e549a-fd37-4839-9f2c-8c0a3c396610Document227 pagesda3e549a-fd37-4839-9f2c-8c0a3c396610Deepika singhNo ratings yet
- NLM 6Document3 pagesNLM 6Ramesh BadamNo ratings yet
- David HumeDocument5 pagesDavid HumeEric StoneNo ratings yet
- Lecture Notes in Nuclear Medicine - Edited Version)Document65 pagesLecture Notes in Nuclear Medicine - Edited Version)Jestia Lyn EngracialNo ratings yet
- Graphing Data: Investigation 2B: Graphical RelationshipsDocument3 pagesGraphing Data: Investigation 2B: Graphical RelationshipsellaNo ratings yet
- Modified Soave-Redlich-Kwong EoS Applied To Mixtures Containing Supercritical CO2Document7 pagesModified Soave-Redlich-Kwong EoS Applied To Mixtures Containing Supercritical CO2Davide Di ZioNo ratings yet
- Narayana - 15!06!2022 - Outgoing SR - Jee Main Model Gtm-10 - QuesDocument22 pagesNarayana - 15!06!2022 - Outgoing SR - Jee Main Model Gtm-10 - QuesShreyas VedantiNo ratings yet
- Area of A Circle: Grade VDocument3 pagesArea of A Circle: Grade VCHar RyNo ratings yet
- Radiography Procedure RNDTDocument27 pagesRadiography Procedure RNDTrashmibetuNo ratings yet
- Ii (Specti I (& (LLTS) A F.Eading NDT: Traiim (GDocument1 pageIi (Specti I (& (LLTS) A F.Eading NDT: Traiim (Gআশার আলোNo ratings yet
- MC Web Mech2 9 2009 PDFDocument2 pagesMC Web Mech2 9 2009 PDFAshleyJaneFuentesNo ratings yet
- Lecture 08 - ME 243 - Helical SpringsDocument14 pagesLecture 08 - ME 243 - Helical Springssalmanalamj5No ratings yet
- Control of A Heat Exchanger Using Takagi-Sugeno Fuzzy ModelDocument6 pagesControl of A Heat Exchanger Using Takagi-Sugeno Fuzzy ModelLuis ZapataNo ratings yet
- ATOICV1 7 1 Limitation of Crystal Field TheoryDocument12 pagesATOICV1 7 1 Limitation of Crystal Field TheoryAYESHA MALIKNo ratings yet
- Tutorial Optics ClassDocument13 pagesTutorial Optics ClassHY ChanNo ratings yet
- Kinetic Theory: Chapter ThirteenDocument7 pagesKinetic Theory: Chapter ThirteenAamerNo ratings yet
- Science8 q1 Mod2 LawsOfMotion FINAL07282020Document30 pagesScience8 q1 Mod2 LawsOfMotion FINAL07282020Cristy VillamorNo ratings yet
- Coordinate Geometry2Document90 pagesCoordinate Geometry2wahida wahabNo ratings yet
- Single Phase Diagram - Real Gases - 003Document36 pagesSingle Phase Diagram - Real Gases - 003JatskinesisNo ratings yet
- Human Eye Complete - Watermarked PDFDocument13 pagesHuman Eye Complete - Watermarked PDFanandatulNo ratings yet
- Ti Keranol Ve 110 424k enDocument3 pagesTi Keranol Ve 110 424k enPoliana PolyNo ratings yet
- Folic Cokic - GNP2020Document18 pagesFolic Cokic - GNP2020Kenan KajosevicNo ratings yet
- Introduction To Boundary Value Problems E-NoteDocument6 pagesIntroduction To Boundary Value Problems E-NotexingmingNo ratings yet
- Unit 1 - Home Life N01: Full name: NGUYỄN KHẮC PHÁT Class: 12a1Document9 pagesUnit 1 - Home Life N01: Full name: NGUYỄN KHẮC PHÁT Class: 12a1Phương ThuNo ratings yet
- Ejercicios Practicos Electricidad 2 EsoDocument7 pagesEjercicios Practicos Electricidad 2 EsoJOSE RAMONNo ratings yet