Professional Documents
Culture Documents
Intro Summary
Uploaded by
Chastine CruzCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Intro Summary
Uploaded by
Chastine CruzCopyright:
Available Formats
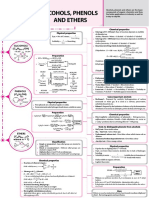
Summary Sheet - Introduction to Chemical Reactivity, Nomenclature, Boiling Points, and Water Solubility http://masterorganicchemistry.
com
Reactant #1 Reactant #2 Product Example Notes The Four Types of Intermolecular Bonding in Organic Chemistry •Boiling point increases with strength of the
intermolecular interactions.
H H Pd/C, H2 HH HH •Water solubility increases with polarity.
Alkene Pd/C + H2 Alkane cis addition (hydrogens go Type of
R R R R on same side of alkene) Name Interaction Found in Example Strength Notes
HCl gives rise to greatest water
Amine Acid Ammonium amines but NOT amides Ionic Attraction Salts NH4 Cl Strongest
R NH2 R NH3Cl between solubility (most polar)
salt (amides are not basic on nitrogen)
point charges δ+ also highest boiling points
O O O
EtNH2 H H
Carboxylic Base Salt
acid (Carboxylate Ph OH Ph O EtNH3 δ−
salt) Attraction between Water, alcohols O
positively charged H carboxylic acids H H 2nd strongest 2nd greatest for effect on
Hydrogen
O O and negatively charged amides, amines water solubility and boiling
MeOH Bonding
O, N or F. points
Carboxylic Alcohol, Ester R OH R OMe Alcohols used as solvent δ−
acid acid, heat H2SO4, Δ O
δ+
ketones, aldehydes,
Attraction between esters, alkyl halides, O δ− Increases as electronegativity
O H2O O This is the reverse of the Dipole- 2nd weakest difference increases
Ester Water, Carboxylic dipole moments etc. - any molecule
above reaction. Dipole
acid, heat acid caused by differences with a strongly δ+ 3rd greatest for effect on water
R OMe H2SO4, Δ R OH in electronegativity electronegative solubilty and boiling points
Here we use water as solvent. element (O, N, F, Cl, Br)
Increases with surface area
(increasing length of carbon
O H2O O Attraction between Hydrocarbons Name Weakest chains)
Ester Water, Carboxylic This is called ester hydrolysis Van Der Waals
base acid or saponification (London forces) temporary dipoles worst for water solubility
R OMe NaOH R OH
(least polar)
best for solubility in non-polar
solvents (e.g. pentane)
R R OH alcohol forms on most
substituted carbon Functional Group Name Example Name
HCl # Carbons Root
Alkene Water, acid Alcohol H (Markovnikoff rule)
R CH2 H2O R C 1 Meth- R– Alkyl Pentane
H2 proceeds through carbocation
2 Eth- –OH Hydroxyl OH Pentanol or pentyl alcohol
R R Cl halide adds to most
HCl substituted carbon 3 Prop-
Alkene Strong acid Alkyl H (Markovnikoff rule) –Cl, –Br, –F, –I Halide Cl Pentyl chloride
R CH2 R C 4 But-
halide H2 proceeds through carbocation
5 Pent- –NH2 Amine NH2 Pentylamine
6 Hex- O Ether O Pentyl methyl ether
Br R R R
Br2 H Results in trans product
Alkene Br2 Dibromide R H 7 Hept-
R R Br –SH Thiol SH Pentane thiol
8 Oct-
R
K2CrO7 C C Alkene Pentene
Alcohol K2CrO7 Carboxylic acid OH O 9 Non-
(primary alcohol) R R
H2O O O
OR ketone OH 10- Dec-
(secondary alcohol) Aldehyde Pentanal
R C H H
R
K2CrO7 O O
OH O Note that secondary alcohols
R R stop at the ketone stage Ketone Butyl methyl ketone
H2O Primary carbon: attached to ONE R C R Me OR 2-pentanone
R R carbon atom
O O
R Secondary: attached to TWO Carboxylic Pentanoic acid
KMnO4 carbon atoms R C OH acid OH
Alcohol KMnO4 Carboxylic acid OH O
(primary alcohol) R R
H2O Tertiary: attached to THREE O O
OH carbon atoms
R C OR Ester OMe Methyl pentanoate
Cl2, hγ Cl Cl Free-radical reaction Quaternary: attached to FOUR
Alkyl chloride O O
Alkane Cl2, hν R Me R CCl3 (number of new C–Cl carbon atoms
Amide N-methyl pentamide
(or peroxides) bonds depends on R C NH2 NHMe
# of equivalents)
Other important nomenclature terms to remember
R R
Br2, FeBr3 Also gives 1,4 (para) product Copyright 2012
Br Br James A.Ashenhurst
Br2, FeCl3 but never 1,3 (meta) product OH R R R
Benzene Aryl bromide Aug 2012, version 1.1
derivative james@masterorganicchemistry
R .com
Phenyl isopropyl
[ox] Trans Cis
R-SH RS–SR (e.g. phenyl e.g. isopropanol 1,2 1,3
Thiol "Oxidant" Disulfide bromide) 1,4
ortho meta
para
You might also like
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- Synthesis of ImidazolesDocument5 pagesSynthesis of ImidazolesMuhammad SalehNo ratings yet
- Chemistry - May 2016Document1 pageChemistry - May 2016Rahique ShuaibNo ratings yet
- Biologically Relevant Polymer Presentation: Lecture 5: Interfacial Polymerizations, Application Example: PolyamidesDocument4 pagesBiologically Relevant Polymer Presentation: Lecture 5: Interfacial Polymerizations, Application Example: PolyamidesKrishna KumarNo ratings yet
- Nature - White - 2011 (Desaturacions) PDFDocument7 pagesNature - White - 2011 (Desaturacions) PDFCarlotaNo ratings yet
- Mechanisms For Final Exam: Electrophilic Aromatic SubstitutionDocument4 pagesMechanisms For Final Exam: Electrophilic Aromatic SubstitutionMarie St. LouisNo ratings yet
- Tabla de OxidacionDocument1 pageTabla de OxidacionMartinez Herrera IsaacNo ratings yet
- Alcohols Phenols and EthersDocument1 pageAlcohols Phenols and EthersNitisha GuptaNo ratings yet
- Course 201N 1 Semester 2006-2007 Inorganic Chemistry Instructor: Jitendra K. BeraDocument10 pagesCourse 201N 1 Semester 2006-2007 Inorganic Chemistry Instructor: Jitendra K. BeraNITU KUMARINo ratings yet
- Named ReactionsDocument15 pagesNamed ReactionsSony mulgundNo ratings yet
- Amino Acid and BiochemistryDocument10 pagesAmino Acid and BiochemistryUNKNOWNNo ratings yet
- 5.2 Alkohol Dari Senyawa KarbonilDocument41 pages5.2 Alkohol Dari Senyawa KarbonilRatnahNo ratings yet
- "Master Organic Chemistry": Anti-Markovnikoff BH H Syn AdditionDocument1 page"Master Organic Chemistry": Anti-Markovnikoff BH H Syn AdditionMoncef AbbesNo ratings yet
- Addition To Alkenes CheatsheetDocument1 pageAddition To Alkenes CheatsheetChinmay MhatreNo ratings yet
- Reactions of Carboxylic Acids - SibiDocument4 pagesReactions of Carboxylic Acids - SibiAmir HussainNo ratings yet
- Hydrocarbons Derivatives - Alkyl Halide - Aryl Halide PDFDocument15 pagesHydrocarbons Derivatives - Alkyl Halide - Aryl Halide PDFAhmed HammadNo ratings yet
- Alkynes Medorg2Document6 pagesAlkynes Medorg2AR LazagaNo ratings yet
- Organic Synthesis. ReductionsDocument64 pagesOrganic Synthesis. ReductionsKartik RanaNo ratings yet
- Ald and Ket Part 1Document3 pagesAld and Ket Part 1Aryan GuptaNo ratings yet
- Chapter 6 PDFDocument13 pagesChapter 6 PDFprince sharmaNo ratings yet
- Consider The Following Anion CH CH CHCHDocument13 pagesConsider The Following Anion CH CH CHCHbobNo ratings yet
- Chem-353-Lecture 2Document10 pagesChem-353-Lecture 2Caleb AsharleyNo ratings yet
- Acid Bases 2018Document2 pagesAcid Bases 2018Siti NorhayatiNo ratings yet
- Wiley's Solomons, Fryhle Snyder Organic Chemistry - 720-742Document23 pagesWiley's Solomons, Fryhle Snyder Organic Chemistry - 720-742vishalgowni8888No ratings yet
- Hydrocarbons: Ella EH EH ÉDocument19 pagesHydrocarbons: Ella EH EH ÉIsabella LopezNo ratings yet
- Mind Map Organic - PDT 2021Document11 pagesMind Map Organic - PDT 2021ROS EZRA HANNY A/P ROSLI MoeNo ratings yet
- 7 Oxidation Ladder 2019 PDFDocument1 page7 Oxidation Ladder 2019 PDFNoel SibyNo ratings yet
- SynrxnsDocument48 pagesSynrxnsRonak MantriNo ratings yet
- CHM 416: Organic SynthesisDocument68 pagesCHM 416: Organic SynthesisSochimaobi EmenikeNo ratings yet
- Aldehyde Ketone and AcidDocument15 pagesAldehyde Ketone and AcidSsNo ratings yet
- Alcohol and Ether BSC 1st YearDocument14 pagesAlcohol and Ether BSC 1st YearSamrina NepalNo ratings yet
- Module 8 Aldehydes and KetonesDocument9 pagesModule 8 Aldehydes and KetonesMaxine AquinoNo ratings yet
- ch18 SummaryDocument1 pagech18 Summaryapi-465421809No ratings yet
- Θεωρία Υβριδισμού Του Ατόμου Του Άνθρακα Του Δημήτρη ΣιάπκαDocument27 pagesΘεωρία Υβριδισμού Του Ατόμου Του Άνθρακα Του Δημήτρη Σιάπκαaggelisgeorge8546No ratings yet
- 20 C N Bond Forming ReactionsDocument15 pages20 C N Bond Forming ReactionsAnonymous XHwkbC7No ratings yet
- Summary Sheet - Functional GroupsDocument1 pageSummary Sheet - Functional GroupsSaidur Mursalin RafterNo ratings yet
- DH AmakaDocument91 pagesDH AmakaazsaNo ratings yet
- Grignard 2Document10 pagesGrignard 2Paolo PepsNo ratings yet
- Grignard PDFDocument12 pagesGrignard PDFDicky LaurentiusNo ratings yet
- Alcohols 2Document4 pagesAlcohols 2Abdullah SalmainNo ratings yet
- Chapter 8 SlidesDocument63 pagesChapter 8 SlidespoojaNo ratings yet
- Grig NardDocument12 pagesGrig Narddhalashutosh413No ratings yet
- 9 Alkenes Alkynes PostDocument26 pages9 Alkenes Alkynes Postapi-3767370No ratings yet
- Grupos Funcionales CompendioDocument2 pagesGrupos Funcionales CompendioMaria ReyesNo ratings yet
- Experiment C: Synthesis of Frambinone by Aldol Condensation and Catalytic HydrogenationDocument6 pagesExperiment C: Synthesis of Frambinone by Aldol Condensation and Catalytic HydrogenationalinaNo ratings yet
- 4 Introductory Organic Chemistry and AlkanesDocument12 pages4 Introductory Organic Chemistry and AlkanesChristina HerculesNo ratings yet
- Carbonyls - Alpha SubstitutionsDocument8 pagesCarbonyls - Alpha SubstitutionsRavi Kiran KoduriNo ratings yet
- Summary Alcohol (Secondary)Document1 pageSummary Alcohol (Secondary)ALIS SUHAIRIN BT ABD GHANI BMNo ratings yet
- Iit Reductions PDFDocument71 pagesIit Reductions PDFAshish SinghNo ratings yet
- Score Booster Chemistry Part 2Document122 pagesScore Booster Chemistry Part 2IGNiTOR 金 GAMINGNo ratings yet
- CHEM F111 General Chemistry: Electrophilic Addition ReactionDocument18 pagesCHEM F111 General Chemistry: Electrophilic Addition ReactionUtkarsh BansalNo ratings yet
- Ch12 Alcohols From Carbonyl CompoundsDocument85 pagesCh12 Alcohols From Carbonyl CompoundsREGINE CUEVASNo ratings yet
- Functional Group Interconversion Scheme PDFDocument1 pageFunctional Group Interconversion Scheme PDFBilal AhmadNo ratings yet
- JB CI 13.1 HalogenoalkanesDocument6 pagesJB CI 13.1 HalogenoalkanesOCRChemistrySaltersNo ratings yet
- Lithium RajendraDocument23 pagesLithium Rajendravijithebest11No ratings yet
- FI-Medicinal Chemistry 1-Smsrt Genap - TerbaruDocument24 pagesFI-Medicinal Chemistry 1-Smsrt Genap - TerbaruSahabat BaljaiNo ratings yet
- Scheme 3: Cyclopalladated Complexes With Optical Resolution of Amino AcidsDocument3 pagesScheme 3: Cyclopalladated Complexes With Optical Resolution of Amino AcidsAfrah MNo ratings yet
- Stereocenters Are Labeled R or SDocument6 pagesStereocenters Are Labeled R or SChastine CruzNo ratings yet
- Analytical Chemistry 1 - Chem Tech 2019Document144 pagesAnalytical Chemistry 1 - Chem Tech 2019Chastine CruzNo ratings yet
- 43691Document58 pages43691NelaNo ratings yet
- MeasurementsDocument41 pagesMeasurementsLyzza Joyce BaylonNo ratings yet
- PDFDocument3 pagesPDFJude GomezNo ratings yet
- Test 1Document9 pagesTest 1Julie Anne CristalesNo ratings yet
- The Freezing Point of Water: Apparatus (Per Group) SafetyDocument54 pagesThe Freezing Point of Water: Apparatus (Per Group) SafetyNermeinKhattabNo ratings yet
- Future Tenses ExerciseDocument5 pagesFuture Tenses Exerciseroxana kwiekNo ratings yet
- Distilling Absinthe - Randy HortonDocument16 pagesDistilling Absinthe - Randy Hortondave100% (1)
- Bar Menu HSRDocument8 pagesBar Menu HSRprashant manhasNo ratings yet
- Beverage Beverage Service Standards: Arm Catering & CL B Operations" Army Catering & Club Operations"Document16 pagesBeverage Beverage Service Standards: Arm Catering & CL B Operations" Army Catering & Club Operations"MARY JOY VILLARUELNo ratings yet
- Shaun of The DeadDocument137 pagesShaun of The DeadJuan PointisNo ratings yet
- Simple and Fractional Distillation - Formal ReportDocument3 pagesSimple and Fractional Distillation - Formal ReportBP Laforteza0% (3)
- Carbohydrates: Organic ChemistryDocument39 pagesCarbohydrates: Organic Chemistryistri kyungsoNo ratings yet
- Beer Bitterness Ratio Chart Bu Gu PDFDocument1 pageBeer Bitterness Ratio Chart Bu Gu PDFadriano70100% (3)
- Coffee MenuDocument6 pagesCoffee MenuChef TronomicsNo ratings yet
- Lesson 4 GlassesDocument6 pagesLesson 4 GlassesGabriel LowerNo ratings yet
- Chemistry Revision Test - No. 5 (Haloalkanes & Haloalkanes & Alcohols, Phenols & Ethers)Document9 pagesChemistry Revision Test - No. 5 (Haloalkanes & Haloalkanes & Alcohols, Phenols & Ethers)Charles Murgasan Charles MurgasanNo ratings yet
- K'S First Case Resumen CompletoDocument3 pagesK'S First Case Resumen CompletoJazmin Martini FernandezNo ratings yet
- The End of SomethingDocument5 pagesThe End of Somethingamy amorNo ratings yet
- Đề Cương 11. 2023Document6 pagesĐề Cương 11. 2023fwsxcsrr67No ratings yet
- Jeopardy Riddles - ShayaanDocument4 pagesJeopardy Riddles - ShayaanShayaan Ali KhimaniNo ratings yet
- 50 Irregular Verb.sDocument2 pages50 Irregular Verb.sjavier cortes diaz0% (1)
- Random Potion TableDocument2 pagesRandom Potion TableRadosław KwiecińskiNo ratings yet
- Retete Anna OslonDocument68 pagesRetete Anna OslonAlexandra Glodan100% (5)
- Coffee Project New 4Document78 pagesCoffee Project New 4SN KateelNo ratings yet
- WWW - Cartiaz.ro: No Questions Quiz 54 AnswersDocument1 pageWWW - Cartiaz.ro: No Questions Quiz 54 AnswersAkshay bhondeNo ratings yet
- Dre Masso - Classic Cocktails at Home - MobileDocument174 pagesDre Masso - Classic Cocktails at Home - MobileDaniel Robles MotaNo ratings yet
- The Butcher of Anderson Station PDF - 1 13Document13 pagesThe Butcher of Anderson Station PDF - 1 13JohnNY_Tex100% (1)
- Diabetes and Insulin Injections: Your Kaiser Permanente Care InstructionsDocument12 pagesDiabetes and Insulin Injections: Your Kaiser Permanente Care InstructionsGrasiela CampbellNo ratings yet
- SHS Eapp Q1 Las WK7 Day1-4Document6 pagesSHS Eapp Q1 Las WK7 Day1-4Cecille Hernando100% (2)
- PDDS Laboratory Midterms ReviewerDocument5 pagesPDDS Laboratory Midterms ReviewerStephanie AlyssonNo ratings yet
- D&A Procedure PDFDocument5 pagesD&A Procedure PDFStefan RaduNo ratings yet
- Soroka Pepsi Marketingmix PDFDocument15 pagesSoroka Pepsi Marketingmix PDFМарияNo ratings yet
- Caribou-Munroes Island Provincial Park: 2119 Three Brooks Road, Caribou, NS - 902-485-6134Document1 pageCaribou-Munroes Island Provincial Park: 2119 Three Brooks Road, Caribou, NS - 902-485-6134mishka123No ratings yet
- Module 2 - WineDocument102 pagesModule 2 - WineWhena RiosNo ratings yet