Professional Documents
Culture Documents
Association of Plasma Biomarkers of Alzheimer Disease With Cognition and Medical Comorbidities in A Biracial Cohort
Uploaded by
Pei-Hao ChenOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Association of Plasma Biomarkers of Alzheimer Disease With Cognition and Medical Comorbidities in A Biracial Cohort
Uploaded by
Pei-Hao ChenCopyright:
Available Formats
RESEARCH ARTICLE
Association of Plasma Biomarkers of Alzheimer
Disease With Cognition and Medical Comorbidities
in a Biracial Cohort
Vijay K. Ramanan, MD, PhD, Jonathan Graff-Radford, MD, Jeremy Syrjanen, MS, Dror Shir, MD, Correspondence
Alicia Algeciras-Schimnich, PhD, John Lucas, PhD, Yuka A. Martens, PhD, Minerva M. Carrasquillo, PhD, Dr. Ramanan
Gregory S. Day, MD, Nilüfer Ertekin-Taner, MD, PhD, Christian Lachner, MD, Floyd B. Willis, MD, ramanan.vijay@mayo.edu
David S. Knopman, MD, Clifford R. Jack, Jr., MD, Ronald C. Petersen, MD, PhD, Prashanthi Vemuri, PhD,
Neill Graff-Radford, MBBCh,* and Michelle M. Mielke, PhD*
®
Neurology 2023;101:e1402-e1411. doi:10.1212/WNL.0000000000207675
Abstract
Background and Objectives
Recent advances in blood-based biomarkers offer the potential to revolutionize the diagnosis and
management of Alzheimer disease (AD), but additional research in diverse populations is critical.
We assessed the profiles of blood-based AD biomarkers and their relationships to cognition and
common medical comorbidities in a biracial cohort.
Methods
Participants were evaluated through the Mayo Clinic Jacksonville Alzheimer Disease Research
Center and matched on age, sex, and cognitive status. Plasma AD biomarkers (β-amyloid
peptide 1–42 [Aβ42/40], plasma tau phosphorylated at position 181 [p-tau181], glial fibrillary
acidic protein [GFAP], and neurofilament light) were measured using the Quanterix SiMoA
HD-X analyzer. Cognition was assessed with the Mini-Mental State Examination. Wilcoxon
rank sum tests were used to assess for differences in plasma biomarker levels by sex. Linear
models tested for associations of self-reported race, chronic kidney disease (CKD), and vascular
risk factors with plasma AD biomarker levels. Additional models assessed for interactions
between race and plasma biomarkers in predicting cognition.
Results
The sample comprised African American (AA; N = 267) and non-Hispanic White (NHW; N =
268) participants, including 69% female participants and age range 43–100 (median 80.2) years.
Education was higher in NHW participants (median 16 vs 12 years, p < 0.001) while APOE e4
positivity was higher in AA participants (43% vs 34%; p = 0.04). We observed no differences in
plasma AD biomarker levels between AA and NHW participants. These results were unchanged
after stratifying by cognitive status (unimpaired vs impaired). Although the p-tau181-cognition
association seemed stronger in NHW participants while the Aβ42/40-cognition association
seemed stronger in AA participants, these findings did not survive after excluding individuals with
CKD. Female participants displayed higher GFAP (177.5 pg/mL vs 157.73 pg/mL; p = 0.002)
and lower p-tau181 (2.62 pg/mL vs 3.28 pg/mL; p = 0.001) levels than male participants. Diabetes
was inversely associated with GFAP levels (β = −0.01; p < 0.001).
Discussion
In a biracial community-based sample of adults, we observed that sex differences, CKD, and
vascular risk factors, but not self-reported race, contributed to variation in plasma AD bio-
markers. Although some prior studies have reported primary effects of race/ethnicity, our
*These authors contributed equally to this work as co-senior authors.
From the Department of Neurology (V.K.R., J.G.-R., D.S., D.S.K., R.C.P.), Department of Quantitative Health Sciences (J.S., R.C.P.), and Department of Laboratory Medicine and
Pathology (A.A.-S.), Mayo Clinic, Rochester, MN; Department of Psychiatry and Psychology (J.L., C.L.), Department of Neuroscience (Y.A.M., M.M.C., G.S.D., N.E.-T.), Department of
Neurology (N.E.-T., C.L., N.G.-R.), and Department of Family Medicine (F.B.W.), Mayo Clinic, Jacksonville, FL; Department of Radiology (C.R.J., P.V.), Mayo Clinic, Rochester, MN; and
Department of Epidemiology and Prevention (M.M.M.), Wake Forest University School of Medicine, Winston-Salem, NC.
Go to Neurology.org/N for full disclosures. Funding information and disclosures deemed relevant by the authors, if any, are provided at the end of the article.
e1402 Copyright © 2023 American Academy of Neurology
Copyright © 2023 American Academy of Neurology. Unauthorized reproduction of this article is prohibited.
Glossary
AA = African American; Aβ = β-amyloid; Aβ40 = Aβ peptide 1–40; Aβ42 = Aβ peptide 1–42; AD = Alzheimer disease; ADRC =
Alzheimer Disease Research Center; BMI = body mass index; CAD = coronary artery disease; CKD = chronic kidney disease;
CU = cognitively unimpaired; DEM = dementia; GFAP = glial fibrillary acidic protein; MCI = mild cognitive impairment;
MMSE = Mini-Mental State Examination; NfL = neurofilament light; NHW = non-Hispanic White; p-tau181 = plasma tau
phosphorylated at position 181; SiMoA = single-molecule array.
results reinforce the need to account for broad-based medical and social determinants of health (including sex, systemic
comorbidities, and other factors) in effectively and equitably deploying plasma AD biomarkers in the general population.
Introduction In this study, we analyzed a large, biracial, community-based
sample with the primary objective to determine whether race/
Several promising blood-based biomarkers of Alzheimer disease ethnicity, sex differences, and medical comorbidities would be
(AD) pathophysiology have emerged in research studies,1–5 and associated with differential profiles of ultrasensitive single-
there is increasing use of these biomarkers for enrollment molecule array (SiMoA)–based plasma AD biomarkers. We
screening and treatment monitoring in interventional trials.6,7 also assessed whether self-reported race would modify the
These developments have created optimism that blood-based associations between plasma AD biomarkers and cognition.
biomarkers may revolutionize diagnosis and management para-
digms for AD, including facilitating noninvasive, cost-effective,
and infrastructure-efficient deployment to augment existing
tools for evaluation of cognitive impairment in the general
Methods
population.8,9 However, there is a strong consensus that addi- Sample Characteristics
tional research is needed to guide appropriate use of blood-based Older adults residing in Jacksonville, Florida, and surrounding
AD biomarkers outside of selective research studies and spe- communities were recruited from patient visits in local clinic
cialized memory clinics.9 practice and through outreach into the community. Enrolled
participants were seen annually through Mayo Clinic Alz-
A major priority for additional research includes broader study heimer Disease Research Center (ADRC) resources and have
of blood-based biomarkers in diverse, heterogeneous pop- been included in several prior cohort studies.16–18 Study visits
ulations that are more representative of real-world clinical included interviews for medical history, detailed neurologic and
practice. Some prior studies have reported that race/ethnicity general physical examination, cognitive assessments (described
may be associated with differences in plasma AD biomarker further), and blood sample collection. Participants or their
levels. One cross-sectional study of individuals with normal surrogates provided written informed consent to participate in
cognition or mild cognitive impairment (MCI) described lower research. Study protocols were approved by the Mayo Clinic
levels of plasma tau phosphorylated at position 181 (p-tau181), Institutional Review Board. Cognitive status for each partici-
β-amyloid (Aβ) peptide 1–42 (Aβ42), and Aβ peptide 1–40 pant (cognitively unimpaired [CU], MCI, and dementia
(Aβ40) in African American (AA) individuals compared with [DEM]) was determined by a multidisciplinary consensus
those in non-Hispanic White (NHW) individuals.10 A different panel incorporating all available information and referencing
study reported lower plasma Aβ40 and higher total tau in established diagnostic criteria.19
cognitively normal Mexican American individuals compared
with those in NHW individuals.11 However, no plasma bio- Demographic, Clinical, and Genetic Data
marker differences (total tau, p-tau181, p-tau217, Aβ42/Aβ40, and Race (AA vs NHW participants) and sex (male vs female par-
neurofilament light [NfL]) by self-reported race were observed ticipants) were determined by self-report on a free-response
in analyses of a separate multiethnic, community-based co- question posed during an in-person interview. Age and years of
hort.12 Another study found that select plasma AD biomarkers education were also ascertained for each participant. The partici-
may perform inconsistently (in predicting brain amyloidosis) pant’s Mini-Mental State Examination (MMSE)20 score was used
across AA and NHW individuals, although sample size and as a measure of cognitive functioning. APOE ɛ4 allele status was
characteristics (e.g., cognitive status, medical comorbidities, assessed through Taqman genotyping assays on stored blood
social determinants of health) may have influenced the re- samples, as previously described.21 Height (in centimeters) and
sults.13 Furthermore, among the general population with AD, weight (in kilograms) for each participant was measured and used
there is wide heterogeneity in social determinants of health and to calculate body mass index (BMI; kg/m2). Cardiovascular
medical comorbidities, all of which may affect the interpretation risk factors (hypertension, diabetes, tobacco usage [current,
of AD biomarkers11,14,15 and thus motivate additional study. former, and never], stroke, coronary artery disease [CAD])
Neurology.org/N Neurology | Volume 101, Number 14 | October 3, 2023 e1403
Copyright © 2023 American Academy of Neurology. Unauthorized reproduction of this article is prohibited.
were determined primarily by medical record abstraction with were flipped (i.e., 30 − MMSE) and log transformed (1 was
supplementing from research charts and National Alzheimer added to everyone’s MMSE score to avoid taking the log of 0,
Coordinating Center database forms when available. Presence which is undefined) to assist with interpretation and to reduce
or absence of chronic kidney disease (CKD) was determined skew in variable distribution. For interaction-based analyses,
by medical record abstraction; specifically, chart search was the primary measure of interest was the p value for the bio-
performed for the terms “chronic kidney disease,” “renal fail- marker × race term within a full factorial model (i.e., including
ure,” “renal,” and “kidney,” and for those participants whose the base terms for the biomarker of interest and race along with
records included those terms, clinical notes and laboratory data all covariates). Data preparation and analyses were conducted
were reviewed to ensure this was a chronic diagnosis rendered using SAS version 9.4 (SAS Institute, Cary, NC) and R version
by health providers before the plasma draw for research. 3.6.2 (R Foundation for Statistical Computing, Vienna, Aus-
tria). Statistical significance was defined as p < 0.05.
SiMoA-Based Plasma AD Biomarker Data
Blood samples were collected for all participants. EDTA Data Availability
plasma fractions were aliquoted after centrifuge and stored at Anonymized data will be available on reasonable request from
−80°C. Plasma AD biomarkers were measured on these stored a qualified investigator in accordance with the Mayo ADRC
samples using the Quanterix SiMoA HD-X analyzer (Quan- data sharing protocol.
terix Corp., Lexington, MA) according to manufacturer’s in-
structions. Plasma β-amyloid (Aβ) peptide 1–42 (Aβ42), Aβ
peptide 1–40 (Aβ40), glial fibrillary acidic protein (GFAP), Results
and NfL were assessed using the Simoa Neurology 4-Plex E
Advantage kit (N4PE, item #103670). p-tau181 was measured Among the 535 adults in the study sample, the median age was
using the Simoa p-tau181 Advantage V2 kit (item #103714), 80.2 years (range 43.2–100.1 years), and 69.3% (N = 371)
which uses a mouse monoclonal antibody (AT270) specific were female participants (Table 1). Most of the participants
for the threonine-181 phosphorylation site of human tau were cognitively unimpaired (61.3%), while 9.3% of partici-
protein.22 Samples were analyzed over 57 runs spanning ap- pants had a clinical diagnosis of MCI and 29.3% had a clinical
proximately 4 months using the same kit lot numbers diagnosis of DEM. Hypertension (71%) was common within
throughout. Interassay variability assessed by the coefficient of the sample. Diabetes (19.3%) and CKD (14.3%) were rela-
variation was <13% for all analytes based on 2 levels of tively less common but observed at frequencies similar to
manufacturer-provided quality controls run in duplicate with those documented in older adults overall.24,25 Presence of
each sample batch. APOE e4 was more common among AA participants (43.2%
vs 34.1%; p = 0.04), while median education levels were
Statistical Analyses higher in NHW participants (16 years vs 12 years; p < 0.001).
For this study, AA and NHW participants were matched 1:1 Hypertension (83.1% vs 58.6%) and diabetes (29.4 vs 9.3%)
on age, sex, and cognitive status; 1 AA participant was ex- were more frequent among AA participants when compared
cluded because of failure of their plasma sample run. Log with NHW participants (p < 0.001). The frequencies of CAD
transformations were applied to plasma p-tau181, GFAP, and or prior stroke were not different between NHW and AA
NfL to lessen the skew in their distributions; the ratio of participants. Although hypertension was more common in
plasma Aβ42/40 (primarily analyzed based on prior work23) female (74.6%) than male (62.7%) participants within the
did not require log transformation. When used as predictors sample (p = 0.006), there were no sex differences in the
in models, all plasma markers were also z standardized to frequencies of diabetes, CAD, prior stroke, or APOE e4 allele
assist with interpretation. For any statistical outliers in plasma presence. Male participants displayed a higher frequency of
biomarker measures (≥3 SDs from the sample mean), anal- CKD than female participants (19.0% vs 12.2%), but this
yses were repeated after excluding outliers to ensure no undue difference was not statistically significant (p = 0.08).
influence on the results. For unadjusted comparisons of var-
iables by sex and race, Wilcoxon rank sum tests were used for As expected, age had robust associations with plasma bio-
continuous measures, and Pearson chi-squared tests were marker levels in all models (βNfL = 0.37; βGFAP = 0.26; βp-tau181
used for categorical measures. Linear models were analyzed = 0.19; βAβ42/40 = −0.004; all p < 0.001). We also observed that
with plasma biomarkers as outcomes and race, CKD, and sex, but not self-reported race/ethnicity, was associated with
vascular disease risk factors (BMI [modeled as a continuous plasma biomarker levels (Figure). Specifically, female partici-
measure] and presence of hypertension, diabetes, tobacco pants displayed higher GFAP (177.5 pg/mL vs 157.73 pg/mL;
usage, stroke, or CAD) as independent predictors in separate p = 0.002) and lower p-tau181 (2.62 pg/mL vs 3.28 pg/mL; p =
models, covarying for age and sex. Linear models were also 0.001) levels than male counterparts. These differences per-
used to assess the association of plasma biomarkers as pre- sisted when the sample was stratified by cognitive status
dictors of cognition (MMSE score) and to test for statistical (cognitively unimpaired vs MCI/DEM) and within a sensitivity
interactions between the plasma biomarkers and race in pre- analysis after removing individuals with CKD. There were no
dicting cognitive performance. For these analyses, age, sex, differences in plasma biomarker (Aβ42/40, p-tau181, GFAP,
and education were included as covariates, and MMSE scores NfL) levels between AA and NHW participants within the
e1404 Neurology | Volume 101, Number 14 | October 3, 2023 Neurology.org/N
Copyright © 2023 American Academy of Neurology. Unauthorized reproduction of this article is prohibited.
sample overall or after stratifying by cognitive status (cogni-
Table 1 Sample Characteristics tively unimpaired vs MCI/DEM). The lack of association of
AA (N = 267) NHW (N = 268)
self-reported race with plasma biomarker levels was confirmed
with linear models including age and sex as covariates
Age (y) 80.2 (44.0–100.1) 80.1 (43.2–99.7)
(Table 2). In post hoc models stratifying by APOE e4 allele
Sex status, marginally nonsignificant associations with race were
Male 82 (30.7) 82 (30.6)
observed among those with an e4 allele, where plasma p-tau181
(β = 0.16, p = 0.09) and GFAP (β = 0.12, p = 0.08) levels were
Female 185 (69.3) 186 (69.4)
higher in NHW participants when compared with those in AA
Education (y) a
12 (2–20) 16 (7–20) participants.
Clinical diagnosis
Several systemic medical comorbidities were associated with
CU 164 (61.4) 164 (61.2)
the plasma biomarkers (Table 2). Presence of diabetes (β =
DEM 78 (29.2) 79 (29.5) −0.19, p < 0.001) and higher BMI values (β = −0.02, p < 0.001)
MCI 25 (9.4) 25 (9.3)
were associated with lower GFAP levels. Higher BMI values
were also associated with lower NfL levels (β = −0.01, p =
b
Presence of an APOE «4 allele
0.007). CKD was associated with higher p-tau181 (β = 0.33, p <
Negative 138 (56.8) 145 (65.9) 0.001) and NfL (β = 0.35, p < 0.001) levels, while prior stroke
Positive 105 (43.2) 75 (34.1)
was associated with higher NfL levels (β = 0.25, p = 0.003). We
observed no association of hypertension, CAD, or tobacco use
BMIc 27.4 (17.0–47.3) 25.4 (17.2–50.0)
(current or former) with plasma AD biomarker profiles. These
CKDd results were similar when imputation was used to account for
Absent 127 (81.9) 208 (88.1)
missing data in some participants.
Present 28 (18.1) 28 (11.9)
We next investigated relationships between the plasma bio-
Diabetes e markers and cognition and whether self-reported race modified
Absent 180 (70.6) 233 (90.7)
these relationships. In the overall sample, higher plasma GFAP
was most strongly associated with worse MMSE (flipped and
Present 75 (29.4) 24 (9.3)
log transformed) score (β = 0.38, p < 0.001), followed by
Hypertensionf p-tau181 (β = 0.29, p < 0.001) and NfL (β = 0.24, p < 0.001).
Absent 45 (16.9) 108 (41.4)
The plasma Aβ42/40 ratio was not associated with cognition in
the overall sample (β = −0.06, p = 0.13). We observed a modest
Present 221 (83.1) 153 (58.6) interaction (p = 0.01) between self-reported race and plasma
Tobacco use g p-tau181 on cognition, wherein the p-tau181-cognition association
seemed stronger in NHW participants (β = 0.41) than in AA
Current 12 (4.8) 6 (2.3)
participants (β = 0.22). However, these findings were attenuated
Former 87 (34.9) 93 (36.2) (p = 0.38) after excluding individuals with CKD. Similarly,
Never 150 (60.2) 158 (61.5) although the Aβ42/40-cognition association seemed stronger in
h
AA participants than in NHW participants (β = −0.19 vs β =
Stroke
−0.002), the statistical interaction between plasma Aβ42/40 and
Absent 195 (89.4) 224 (93.3) self-reported race was attenuated (p = 0.02 vs p = 0.15) after
Present 23 (10.6) 16 (6.7) excluding individuals with CKD. This interaction was also no
longer statistically significant if an Aβ42/40 outlier was removed.
CADi
Absent 130 (82.3) 200 (83.3)
Present 28 (17.7) 40 (16.7) Discussion
Abbreviations: AA = African American; BMI = body mass index; CAD = cor- Developments in blood-based biomarkers have great po-
onary artery disease; CKD = chronic kidney disease; CU = cognitively
unimpaired; DEM = dementia; MCI = mild cognitive impairment; NHW = non- tential for noninvasive and resource-efficient utilization in
Hispanic White. the general population. A major barrier to wider deployment
Values displayed as median (range) or n (%).
a
Missing for 23 participants (16 AA, 7 NHW). of available assays at this time involves the relative paucity of
b
Missing for 72 participants (24 AA, 48 NHW). data on how these blood-based biomarkers perform in rep-
c
Missing for 221 participants (159 AA, 62 NHW).
d
Missing for 144 participants (112 AA, 32 NHW). resentative populations that better reflect real-world clinical
e
Missing for 23 participants (12 AA, 11 NHW).
f
Missing for 8 participants (1 AA, 7 NHW).
practice. In such settings, systemic medical conditions and
g
h
Missing for 29 participants (18 AA, 11 NHW). social determinants of health would be expected to have
Missing for 77 participants (49 AA, 27 NHW).
i
Missing for 137 participants (109 AA, 28 NHW). different profiles compared with selective research studies
and specialty memory clinics.9 This study of a large, biracial,
Neurology.org/N Neurology | Volume 101, Number 14 | October 3, 2023 e1405
Copyright © 2023 American Academy of Neurology. Unauthorized reproduction of this article is prohibited.
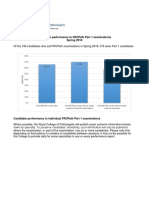
Figure Profiles of Plasma Biomarkers of Alzheimer Disease by Sex and Self-Reported Race
Analyses were performed on a biracial sample matched on age, sex, and clinical diagnosis. Box plots display the distributions of plasma biomarker (Aβ42/40, GFAP,
NfL, and p-tau181) levels among female vs male participants (A) and among AA vs NHW participants (B). Female participants displayed higher GFAP and lower p-
tau181 levels than male participants, differences that persisted after excluding individuals with CKD. No differences by self-reported race were observed for any
plasma biomarker. AA = African American; Aβ = β-amyloid; Aβ40 = Aβ peptide 1–40; Aβ42 = Aβ peptide 1–42; CKD = chronic kidney disease; GFAP = glial fibrillary
acidic protein; NfL = neurofilament light; NHW = non-Hispanic White; p-tau181 = plasma tau phosphorylated at position 181.
community-based sample of adults provides evidence that AD biomarkers and therefore need to be accounted for in the
sex differences and systemic medical comorbidities, but not development of reference ranges and guidance on clinical
self-reported race/ethnicity, influence variation in plasma interpretation.
e1406 Neurology | Volume 101, Number 14 | October 3, 2023 Neurology.org/N
Copyright © 2023 American Academy of Neurology. Unauthorized reproduction of this article is prohibited.
Table 2 Linear Models Predicting Plasma Alzheimer Disease Biomarkers
Biomarker Independent variablea Nb Model R2 β (95% CI) p Value
Aβ42/40 Race (self-reported) 531 0.05 0.0002 (−0.003 to 0.003) 0.91
Aβ42/40 BMI 311 0.04 0.0003 (0.0001 to 0.0006) 0.02
Aβ42/40 CKD 388 0.06 0.004 (−0.001 to 0.009) 0.12
Aβ42/40 Diabetes 508 0.05 −0.001 (−0.004 to 0.003) 0.65
Aβ42/40 Hypertension 523 0.05 −0.00003 (−0.003 to 0.003) 0.99
Aβ42/40 Tobacco use (current) 503 0.05 0.001 (−0.007 to 0.008) 0.77
Aβ42/40 Tobacco use (former) 503 0.05 −0.001 (−0.009 to 0.007) 0.84
Aβ42/40 CAD 395 0.06 0.001 (−0.004 to 0.005) 0.75
Aβ42/40 Stroke 455 0.06 0.001 (−0.005 to 0.006) 0.85
GFAPc Race (self-reported) 534 0.22 −0.03 (−0.11 to 0.05) 0.49
GFAP BMI 314 0.29 −0.02 (−0.03 to −0.01) <0.001
GFAP CKD 391 0.23 0.10 (−0.04 to 0.24) 0.16
GFAP Diabetes 511 0.23 −0.19 (−0.29 to −0.08) <0.001
GFAP Hypertension 526 0.22 −0.01 (−0.10 to 0.08) 0.90
GFAP Tobacco use (current) 506 0.23 0.03 (−0.20 to 0.26) 0.78
GFAP Tobacco use (former) 506 0.23 0.10 (−0.12 to 0.33) 0.37
GFAP CAD 398 0.23 0.05 (−0.08 to 0.17) 0.47
GFAP Stroke 458 0.22 0.02 (−0.13 to 0.17) 0.80
c
NfL Race (self-reported) 534 0.30 −0.03 (−0.11 to 0.06) 0.57
NfL BMI 314 0.37 −0.01 (−0.02 to −0.004) 0.007
NfL CKD 391 0.36 0.35 (0.21 to 0.49) <0.001
NfL Diabetes 511 0.29 0.11 (−0.002 to 0.22) 0.05
NfL Hypertension 526 0.30 0.06 (−0.04 to 0.16) 0.22
NfL Tobacco use (current) 506 0.30 −0.08 (−0.33 to 0.17) 0.52
NfL Tobacco use (former) 506 0.30 −0.11 (−0.35 to 0.14) 0.40
NfL CAD 398 0.33 0.05 (−0.08 to 0.18) 0.43
NfL Stroke 458 0.32 0.25 (0.08 to 0.41) 0.003
p-tau181c Race (self-reported) 532 0.10 0.03 (−0.07 to 0.13) 0.54
p-tau181 BMI 312 0.12 −0.01 (−0.02 to 0.003) 0.14
p-tau181 CKD 389 0.15 0.33 (0.17 to 0.49) <0.001
p-tau181 Diabetes 509 0.10 0.02 (−0.11 to 0.15) 0.76
p-tau181 Hypertension 524 0.10 0.08 (−0.03 to 0.19) 0.16
p-tau181 Tobacco use (current) 503 0.10 0.04 (−0.24 to 0.32) 0.80
p-tau181 Tobacco use (former) 503 0.10 0.10 (−0.18 to 0.37) 0.50
p-tau181 CAD 396 0.13 0.14 (−0.01 to 0.28) 0.07
p-tau181 Stroke 456 0.11 0.07 (−0.12 to 0.26) 0.50
Abbreviations: Aβ = β-amyloid; Aβ40 = Aβ peptide 1–40; Aβ42 = Aβ peptide 1–42; BMI = body mass index; CAD = coronary artery disease; CKD = chronic kidney
disease; GFAP = glial fibrillary acidic protein; NfL = neurofilament light; p-tau181 = plasma tau phosphorylated at position 181.
a
All models included age and sex as covariates, and the outcome measures (plasma biomarkers) were log transformed (except for Aβ42/40); models for
tobacco use had a reference level of never.
b
Number of participants included (having nonmissing data for all variables) in each independent model.
c
Unit of measure pg/mL.
Neurology.org/N Neurology | Volume 101, Number 14 | October 3, 2023 e1407
Copyright © 2023 American Academy of Neurology. Unauthorized reproduction of this article is prohibited.
A prior study of adults from the greater Atlanta metropolitan comorbidities11,14 will be critical in developing reference ranges
area reported lower levels of plasma Aβ40, Aβ42, p-tau181, and and cut points for plasma AD biomarkers.
NfL in AA individuals compared with those in NHW individ-
uals.10 That study sample consisted predominantly of individ- This study has several limitations. We analyzed self-reported
uals with MCI and those in a younger age range than ours. In biological sex as a variable of interest in relation to plasma
addition, that study did not match on age (AA participants were biomarker levels. However, the participant data in this study
younger than NHW participants) and did not consider CKD, may not be able to fully disambiguate sex from gender and
which is known to elevate plasma biomarker levels.11,14,26 An- may not adequately delineate individuals who are transgender
other study identified lower plasma Aβ42/40 levels in Mexican or gender nonbinary. In addition, the information on self-
American participants compared with those in NHW partici- reported race obtained for study participants may be in-
pants, but included similar limitations of clinical diagnostic sufficient for individuals who have multiracial backgrounds or
range (analyzing only cognitively unimpaired individuals), who originate from geographic areas where different racial/
without age or sex matching.11 We previously identified ele- ethnic descriptors are used. While it is advantageous that the
vated plasma total tau levels in AA individuals with AD com- SiMoA platform used in this study is available commer-
pared with cognitively unimpaired control participants, similar cially now, other emerging plasma assays including mass
to findings reported in other ethnoracial groups.21 spectrometry–based measures of Aβ42/405,37,38 and assess-
ments of alternative isoforms of p-tau (e.g., p-tau217 and p-
Despite identifying strong associations with demographic tau231)2,3,26,39 may have superior accuracy in reflecting the
factors (age and sex) and medical comorbidities (e.g., CKD presence of AD pathophysiology, which could affect analyses
and diabetes), we observed no differences in plasma AD of race/ethnicity differences. It is also possible that the ap-
biomarker levels between AA and NHW participants in our proaches we used to obtain diagnoses of CKD and cardio-
sample. These findings did not differ based on cognitive sta- vascular risk factors may not match the true frequency of these
tus. We also examined for APOE e4–specific effects given the conditions assessed by other methods. Relevant for CKD, one
existence of population-level variation in this allele (i.e., more prior study found associations of plasma creatinine (as a
common but less penetrant among AA individuals compared quantitative measure of renal function) with plasma GFAP,
with NHW individuals27,28) and its association with plasma NfL, and p-tau levels, though observed only a modest impact
biomarker levels in previous studies.21,29 Among APOE e4 of creatinine levels toward prediction of other outcomes (e.g.,
carriers in our study, there was a trend toward lower plasma p- corresponding CSF protein levels, subsequent development
tau181 and GFAP levels among AA participants, mirroring of dementia).40 However, that study sample was lacking in
data on CSF biomarkers from an independent sample30 and racial and ethnic diversity and did not include individuals with
parallel literature on amyloid PET.31 Taken together with dementia. Additional medical comorbidities not assessed in
findings from Schindler et al.13 and Brickman et al.,12 our our analyses may also influence plasma biomarker profiles.
results suggest that inconsistencies in plasma AD biomarker
performance in the population have multiple underlying The cross-sectional nature of this study is also a limitation
contributors, including meaningful differences by biomarker because our analyses were not designed to assess the down-
assay, variation in systemic medical and social determinants of stream longitudinal consequences of plasma biomarker levels.
health, and individualized profiles of AD genetic risk factors. Furthermore, although the use of MMSE score as the target
cognitive outcome in this work facilitated maximizing of our
In our study, individuals with diabetes had lower plasma GFAP sample size (for optimal statistical power) and is reflective of a
levels compared with those without. There are some limitations cognitive test administered in typical clinical practice, we
to interpreting this association, including the relatively lower cannot rule out that more detailed measures from a neuro-
frequency of diabetes in NHW participants (9%) compared with psychological test battery restricted to specific etiologic di-
that in AA participants (29%) in this cohort and the fact that we agnoses (e.g., Alzheimer dementia vs vascular dementia) may
did not distinguish between types of diabetes (e.g., type 1 vs type have yielded different results. The associations of diabetes and
2). Blood-based measures of GFAP are hypothesized to be BMI with GFAP levels will additionally benefit from valida-
markers of CNS inflammation and reactive astrogliosis.32 Several tion in larger independent samples. Future work is also
studies have demonstrated associations of plasma GFAP with planned in this cohort to add complementary in vivo bio-
brain amyloidosis3,33 and vascular disease pathology.32,34 Al- markers (neuroimaging and CSF) and postmortem neuro-
though the mechanism behind the diabetes-GFAP association pathology along with longitudinal clinical and biomarker
cannot be determined directly from our dataset, rodent models measures. In summary, this study of a biracial sample rein-
have demonstrated decreased brain GFAP expression in un- forces the need to systematically account for medical and
treated diabetic rats,35 suggesting insulin dysregulation may social determinants of health to maximize the clinical utility of
plausibly influence GFAP levels. In addition, a recent study in a plasma AD biomarkers in the general population.
small cohort identified a similar negative correlation between
BMI and plasma levels of GFAP and NfL.36 More broadly, Acknowledgment
these results support that accounting for the presence of di- The authors thank the study participants and the staff for
abetes and other vascular risk factors and systemic medical the Mayo Clinic Alzheimer Disease Research Center (ADRC)
e1408 Neurology | Volume 101, Number 14 | October 3, 2023 Neurology.org/N
Copyright © 2023 American Academy of Neurology. Unauthorized reproduction of this article is prohibited.
and the community advisory board and action network of the speaker fees from Miller Medical Communications, Inc.
Mayo Clinic Florida ADRC for their assistance in neighbor- and receives research support from the NIH. N. Graff-
hood outreach and engagement in support of participant Radford receives research support from the NIH. M.M.
recruitment. Mielke has served on scientific advisory boards and/or has
consulted for Biogen, LabCorp, Lilly, Merck, Siemens
Study Funding Healthineers, and Sunbird Bio and receives grant support
This work was supported by NIH grants RF1 AG069052, RF1 from the NIH and Department of Defense. The other au-
AG077386, RF1 AG079397, U01 AG006786, R01 NS097495, thors report no disclosures. Go to Neurology.org/N for full
R01 AG56366, P50 AG016574, P30 AG062677, R37 disclosures.
AG011378, R01 AG041851, C06 RR018898, RF AG051504,
U01 AG046139, AG061796, and R01 AG034676; the GHR Publication History
Foundation; the Alexander Family Alzheimer Disease Research Received by Neurology February 3, 2023. Accepted in final form
Professorship of the Mayo Clinic; the Alzheimer Association; June 6, 2023. Submitted and externally peer reviewed. The handling
the Mayo Foundation for Medical Education and Research; the editor was Associate Editor Linda Hershey, MD, PhD, FAAN.
Liston Award; the Elsie and Marvin Dekelboum Family
Foundation; the Schuler Foundation; and grants from the
Florida Department of Health Ed and Ethel Moore Alzheimer Appendix Authors
Disease Research Program (FL 8AZ08, 5AZ03, and 7AZ07). Name Location Contribution
Vijay K. Department of Neurology, Drafting/revision of the
Disclosure Ramanan, Mayo Clinic, Rochester, MN article for content, including
V.K. Ramanan receives research support from the NIH and MD, PhD medical writing for content;
study concept or design; and
the Mangurian foundation for Lewy body disease research. J. analysis or interpretation of
Graff-Radford receives research support from the NIH. G.S. data
Day’s research is supported by the NIH (K23AG064029, Jonathan Department of Neurology, Drafting/revision of the
U01AG057195, and U19AG032438), the Alzheimer Associ- Graff- Mayo Clinic, Rochester, MN article for content,
Radford, MD including medical writing for
ation, and Chan Zuckerberg Initiative; he serves as a consul- content; major role in the
tant for Parabon Nanolabs Inc., as a Topic Editor (Dementia) acquisition of data; study
for DynaMed (EBSCO), and as the Clinical Director of the concept or design; and
analysis or interpretation of
Anti-NMDA Receptor Encephalitis Foundation (Inc., Can- data
ada; uncompensated); he is the coproject PI for a clinical trial
Jeremy Department of Quantitative Drafting/revision of the
in anti-NMDAR encephalitis, which receives support from Syrjanen, MS Health Sciences, Mayo Clinic, article for content, including
Horizon Pharmaceuticals; he has developed educational ma- Rochester, MN medical writing for content;
analysis or interpretation of
terials for PeerView Media, Inc., and Continuing Education, data
Inc.; he owns stock in ANI pharmaceuticals; and his in-
Dror Shir, MD Department of Neurology, Drafting/revision of the
stitution has received support from Eli Lilly for his de- Mayo Clinic, Rochester, MN article for content, including
velopment and participation in an educational event medical writing for content;
major role in the acquisition
promoting early diagnosis of symptomatic Alzheimer disease. of data
N. Ertekin-Taner receives research support from the NIH. C.
Alicia Department of Laboratory Drafting/revision of the
Lachner receives research support from the NIH (P30- Algeciras- Medicine and Pathology, article for content, including
AG062677 Research and Education Core). D.S. Knopman Schimnich, Mayo Clinic, Rochester, MN medical writing for content;
PhD major role in the acquisition
serves on a Data Safety Monitoring Board for the DIAN study, of data
serves on a Data Safety Monitoring Board for Biogen but
receives no personal compensation, is an investigator in John Lucas, Department of Psychiatry Drafting/revision of the
PhD and Psychology, Mayo Clinic, article for content, including
clinical trials sponsored by Biogen, Lilly Pharmaceuticals, and Jacksonville, FL medical writing for content;
the University of Southern California, and serves as a con- major role in the acquisition
of data
sultant for Roche, Samus Therapeutics, Third Rock, and
Alzeca Biosciences but receives no personal compensation. Yuka A. Department of Drafting/revision of the
Martens, Neuroscience, Mayo Clinic, article for content, including
C.R. Jack serves on an independent data monitoring board for PhD Jacksonville, FL medical writing for content;
Roche, has served as a speaker for Eisai, and consulted for major role in the acquisition
of data
Biogen, but he receives no personal compensation from any
commercial entity; he receives research support from the Minerva M. Department of Drafting/revision of the
Carrasquillo, Neuroscience, Mayo Clinic, article for content,
NIH and the Alexander Family Alzheimer Disease Re- PhD Jacksonville, FL including medical writing for
search Professorship of the Mayo Clinic. R.C. Petersen content
serves as a consultant for Roche, Inc., Merck Inc., and Gregory S. Department of Drafting/revision of the
Biogen, Inc., serves on the Data Safety Monitoring Board Day, MD Neuroscience, Mayo Clinic, article for content,
for Genentech, Inc., and receives royalty from Oxford Jacksonville, FL including medical writing
for content
University Press and UpToDate. P. Vemuri received
Continued
Neurology.org/N Neurology | Volume 101, Number 14 | October 3, 2023 e1409
Copyright © 2023 American Academy of Neurology. Unauthorized reproduction of this article is prohibited.
tomography amyloid positivity. Alzheimers Dement. 2022;19(3):956-966. doi:
10.1002/alz.12697
Appendix (continued)
6. Sperling R, Johnson K, Zhou J, et al. Introduction of plasma biomarker screening for the
AHEAD 3 45 study. Clinical Trials on Alzheimer’s Disease Taskforce Meeting; No-
Name Location Contribution
vember 29-December 2, 2022; San Francisco, California.
7. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease.
Nilüfer Department of Drafting/revision of the N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
Ertekin- Neuroscience, and article for content, including 8. Schindler SE. Fluid biomarkers in dementia diagnosis. Continuum (Minneap Minn).
Taner, MD, Department of Neurology, medical writing for content; 2022;28(3):822-833. doi:10.1212/con.0000000000001083
PhD Mayo Clinic, Jacksonville, FL major role in the acquisition 9. Hansson O, Edelmayer RM, Boxer AL, et al. The Alzheimer’s Association appropriate
of data use recommendations for blood biomarkers in Alzheimer’s disease. Alzheimers
Dement. 2022;18(12):2669-2686. doi:10.1002/alz.12756
Christian Department of Psychiatry Drafting/revision of the 10. Hajjar I, Yang Z, Okafor M, et al. Association of plasma and cerebrospinal fluid
Lachner, MD and Psychology, and article for content, including Alzheimer disease biomarkers with race and the role of genetic ancestry, vascular
Department of Neurology, medical writing for content; comorbidities, and neighborhood factors. JAMA Netw Open. 2022;5(10):e2235068.
Mayo Clinic, Jacksonville, FL major role in the acquisition doi:10.1001/jamanetworkopen.2022.35068
of data 11. O’Bryant SE, Petersen M, Hall J, Johnson LA, Team HHS. Medical comorbidities
and ethnicity impact plasma Alzheimer’s disease biomarkers: important consider-
Floyd B. Department of Family Drafting/revision of the ations for clinical trials and practice. Alzheimers Dement. 2023;19(1):36-43. doi:
Willis, MD Medicine, Mayo Clinic, article for content, including 10.1002/alz.12647
Jacksonville, FL medical writing for content; 12. Brickman AM, Manly JJ, Honig LS, et al. Plasma p-tau181, p-tau217, and other blood-
major role in the acquisition based Alzheimer’s disease biomarkers in a multi-ethnic, community study. Alzheimers
of data Dement. 2021;17(8):1353-1364. doi:10.1002/alz.12301
13. Schindler SE, Karikari TK, Ashton NJ, et al. Effect of race on prediction of brain
David S. Department of Neurology, Drafting/revision of the amyloidosis by plasma Aβ42/Aβ40, phosphorylated tau, and neurofilament light.
Knopman, Mayo Clinic, Rochester, MN article for content, including Neurology. 2022;99(3):e245-e257. doi:10.1212/wnl.0000000000200358
MD medical writing for content; 14. Syrjanen JA, Campbell MR, Algeciras-Schimnich A, et al. Associations of amyloid and
major role in the acquisition neurodegeneration plasma biomarkers with comorbidities. Alzheimers Dement. 2022;
of data 18(6):1128-1140. doi:10.1002/alz.12466
15. Gottesman RF, Schneider ALC, Rawlings A, et al. Disparities in dementia and AD
Clifford R. Department of Radiology, Drafting/revision of the biomarkers in the ARIC study: the important contribution of social determinants of
Jack Jr., MD Mayo Clinic, Rochester, MN article for content, including health. Alzheimers Dement. 2021;17(S5):e052844. doi:10.1002/alz.052844
medical writing for content; 16. N’Songo A, Carrasquillo MM, Wang X, et al. African American exome sequencing
major role in the acquisition identifies potential risk variants at Alzheimer disease loci. Neurol Genet. 2017;3(2):
of data e141. doi:10.1212/nxg.0000000000000141
17. Conway OJ, Carrasquillo MM, Wang X, et al. ABI3 and PLCG2 missense variants as
Ronald C. Department of Neurology, Drafting/revision of the risk factors for neurodegenerative diseases in Caucasians and African Americans. Mol
Petersen, and Department of article for content, including Neurodegener. 2018;13(1):53. doi:10.1186/s13024-018-0289-x
MD, PhD Quantitative Health medical writing for content; 18. N’Songo A, Carrasquillo MM, Wang X, et al. Comprehensive screening for disease
Sciences, Mayo Clinic, major role in the acquisition risk variants in early-onset Alzheimer’s disease genes in African Americans iden-
Rochester, MN of data tifies novel PSEN variants. J Alzheimers Dis. 2017;56(4):1215-1222. doi:10.3233/
jad-161185
Prashanthi Department of Radiology, Drafting/revision of the 19. Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive im-
Vemuri, PhD Mayo Clinic, Rochester, MN article for content, including pairment is higher in men. The Mayo Clinic Study of Aging. Neurology. 2010;75(10):
medical writing for content; 889-897. doi:10.1212/wnl.0b013e3181f11d85
major role in the acquisition 20. Cockrell JR, Folstein MF. Mini-mental state examination (MMSE). Psychopharmacol
of data; study concept or Bull. 1988;24(4):689-692.
design; and analysis or 21. Deniz K, Ho CCG, Malphrus KG, et al. Plasma biomarkers of Alzheimer’s dis-
interpretation of data ease in African Americans. J Alzheimers Dis. 2021;79(1):323-334. doi:10.3233/
jad-200828
Neill Graff- Department of Neurology, Drafting/revision of the 22. Karikari TK, Pascoal TA, Ashton NJ, et al. Blood phosphorylated tau 181 as a bio-
Radford, Mayo Clinic, Jacksonville, FL article for content, including marker for Alzheimer’s disease: a diagnostic performance and prediction modelling
MBBCh medical writing for content; study using data from four prospective cohorts. Lancet Neurol. 2020;19(5):422-433.
major role in the acquisition doi:10.1016/s1474-4422(20)30071-5
of data; and study concept 23. Doecke JD, Perez-Grijalba V, Fandos N, et al. Total Aβ42/Aβ40ratio in plasma predicts
or design amyloid-PET status, independent of clinical AD diagnosis. Neurology. 2020;94(15):
e1580-e1591. doi:10.1212/wnl.0000000000009240
Michelle M. Department of Drafting/revision of the 24. Centers for Disease Control and Prevention. National Diabetes Statistics Report
Mielke, PhD Epidemiology and article for content, including website [online]. Accessed January 25, 2023. cdc.gov/diabetes/data/statistics-re-
Prevention, Wake Forest medical writing for content; port/index.html.
University School of major role in the acquisition 25. Centers for Disease Control and Prevention. Chronic Kidney Disease Surveillance
Medicine, Winston-Salem, of data; study concept or System website [online]. Accessed January 25, 2023. nccd.cdc.gov/ckd/default.aspx.
NC design; and analysis or 26. Mielke MM, Dage JL, Frank RD, et al. Performance of plasma phosphorylated tau 181
interpretation of data and 217 in the community. Nat Med. 2022;28(7):1398-1405. doi:10.1038/s41591-022-
01822-2
27. Logue MW, Schu M, Vardarajan BN, et al. A comprehensive genetic association study
of Alzheimer disease in African Americans. Arch Neurol. 2011;68(12):1569-1579. doi:
10.1001/archneurol.2011.646
References 28. Rajan KB, McAninch EA, Wilson RS, Weuve J, Barnes LL, Evans DA. Race, APOEɛ4,
1. Ashton NJ, Janelidze S, Mattsson-Carlgren N, et al. Differential roles of Aβ42/40, p- and long-term cognitive trajectories in a biracial population sample. J Alzheimers Dis.
tau231 and p-tau217 for Alzheimer’s trial selection and disease monitoring. Nat Med. 2019;72(1):45-53. doi:10.3233/jad-190538
2022;28(12):2555-2562. doi:10.1038/s41591-022-02074-w 29. Stevenson-Hoare J, Heslegrave A, Leonenko G, et al. Plasma biomarkers and genetics
2. Janelidze S, Bali D, Ashton NJ, et al. Head-to-head comparison of 10 plasma phospho- in the diagnosis and prediction of Alzheimer’s disease. Brain. 2022;146(2):690-699.
tau assays in prodromal Alzheimer’s disease. Brain. 2022;146(4):1592-1601. doi: doi:10.1093/brain/awac128
10.1093/brain/awac333 30. Morris JC, Schindler SE, McCue LM, et al. Assessment of racial disparities in bio-
3. Mila-Aloma M, Ashton NJ, Shekari M, et al. Plasma p-tau231 and p-tau217 as state markers for Alzheimer disease. JAMA Neurol. 2019;76(3):264-273. doi:10.1001/
markers of amyloid-beta pathology in preclinical Alzheimer’s disease. Nat Med. 2022; jamaneurol.2018.4249
28(9):1797-1801. doi:10.1038/s41591-022-01925-w 31. Deters KD, Napolioni V, Sperling RA, et al. Amyloid PET imaging in self-identified non-
4. Smirnov DS, Ashton NJ, Blennow K, et al. Plasma biomarkers for Alzheimer’s disease Hispanic Black participants of the Anti-Amyloid in Asymptomatic Alzheimer’s Disease
in relation to neuropathology and cognitive change. Acta Neuropathol. 2022;143(4): (A4) study. Neurology. 2021;96(11):e1491-e1500. doi:10.1212/wnl.0000000000011599
487-503. doi:10.1007/s00401-022-02408-5 32. Elahi FM, Casaletto KB, La Joie R, et al. Plasma biomarkers of astrocytic and neuronal
5. Zicha S, Bateman RJ, Shaw LM, et al. Comparative analytical performance of multiple dysfunction in early- and late-onset Alzheimer’s disease. Alzheimers Dement. 2020;
plasma Aβ42 and Aβ40 assays and their ability to predict positron emission 16(4):681-695. doi:10.1016/j.jalz.2019.09.004
e1410 Neurology | Volume 101, Number 14 | October 3, 2023 Neurology.org/N
Copyright © 2023 American Academy of Neurology. Unauthorized reproduction of this article is prohibited.
33. Benedet AL, Milà-Alomà M, Vrillon A, et al. Differences between plasma and cerebro- 37. Schindler SE, Bollinger JG, Ovod V, et al. High-precision plasma beta-amyloid 42/40
spinal fluid glial fibrillary acidic protein levels across the Alzheimer Disease Continuum. predicts current and future brain amyloidosis. Neurology. 2019;93(17):e1647-e1659.
JAMA Neurol. 2021;78(12):1471-1483. doi:10.1001/jamaneurol.2021.3671 doi:10.1212/wnl.0000000000008081
34. Shir D, Graff-Radford J, Hofrenning EI, et al. Association of plasma glial fibrillary acidic protein 38. Janelidze S, Teunissen CE, Zetterberg H, et al. Head-to-head comparison of 8 plasma
(GFAP) with neuroimaging of Alzheimer’s disease and vascular pathology. Alzheimers Dement. amyloid-beta 42/40 assays in Alzheimer disease. JAMA Neurol. 2021;78(11):
2022;14(1):e12291. doi:10.1002/dad2.12291 1375-1382. doi:10.1001/jamaneurol.2021.3180
35. Coleman ES, Dennis JC, Braden TD, Judd RL, Posner P. Insulin treatment pre- 39. Thijssen EH, La Joie R, Strom A, et al. Plasma phosphorylated tau 217 and phos-
vents diabetes-induced alterations in astrocyte glutamate uptake and GFAP con- phorylated tau 181 as biomarkers in Alzheimer’s disease and frontotemporal lobar
tent in rats at 4 and 8 weeks of diabetes duration. Brain Res. 2010;1306:131-141. degeneration: a retrospective diagnostic performance study. Lancet Neurol. 2021;
doi:10.1016/j.brainres.2009.10.005 20(9):739-752. doi:10.1016/s1474-4422(21)00214-3
36. Rebelos E, Rissanen E, Bucci M, et al. Circulating neurofilament is linked with morbid 40. Pichet Binette A, Janelidze S, Cullen N, et al. Confounding factors of Alzheimer’s
obesity, renal function, and brain density. Sci Rep. 2022;12(1):7841. doi:10.1038/ disease plasma biomarkers and their impact on clinical performance. Alzheimers
s41598-022-11557-2 Dement. 2022;19(4):1403-1414. doi:10.1002/alz.12787
Neurology.org/N Neurology | Volume 101, Number 14 | October 3, 2023 e1411
Copyright © 2023 American Academy of Neurology. Unauthorized reproduction of this article is prohibited.
You might also like
- Satoh 2012Document9 pagesSatoh 2012MICHAEL NUGROHONo ratings yet
- Genetic Susceptibility To Cardiovascular Disease in Patients On Kidney Dialysis - Full Text View - ClinicalTrials - GovDocument5 pagesGenetic Susceptibility To Cardiovascular Disease in Patients On Kidney Dialysis - Full Text View - ClinicalTrials - GovAlexandra AvanuNo ratings yet
- Original Investigation A Meta-Analysis of The Association of Estimated GFR, Albuminuria, Age, Race, and Sex With Acute Kidney InjuryDocument11 pagesOriginal Investigation A Meta-Analysis of The Association of Estimated GFR, Albuminuria, Age, Race, and Sex With Acute Kidney Injurysiska tiaraNo ratings yet
- Genome-Wide Association Study of Serum Metabolites in The African American Study of Kidney Disease and HypertensionDocument21 pagesGenome-Wide Association Study of Serum Metabolites in The African American Study of Kidney Disease and Hypertensionsengermartin6No ratings yet
- UsingADNI 2021 VeitchDocument34 pagesUsingADNI 2021 VeitchnayaddouaihyNo ratings yet
- Vitamin D and Telomerase 5Document5 pagesVitamin D and Telomerase 5Muhammad AL Farisi SutrisnoNo ratings yet
- FulltextDocument9 pagesFulltextheheNo ratings yet
- Nihms 98009Document19 pagesNihms 98009folkloriantaroNo ratings yet
- Kidney Function and Cognitive ImpairmentDocument15 pagesKidney Function and Cognitive ImpairmentdenisNo ratings yet
- Genetic Risk, Adherence To A Healthy Lifestyle, and Coronary DiseaseDocument10 pagesGenetic Risk, Adherence To A Healthy Lifestyle, and Coronary DiseaseNurul Falah KalokoNo ratings yet
- Martini2012 PDFDocument10 pagesMartini2012 PDFSofia ImaculataNo ratings yet
- The Alzheimer's Disease Neuroimaging Initiative - ArticleDocument68 pagesThe Alzheimer's Disease Neuroimaging Initiative - ArticleacbgdvNo ratings yet
- Hepatic Gene Networks in Morbidly Obese Patients With Nonalcoholic Fatty Liver DiseaseDocument12 pagesHepatic Gene Networks in Morbidly Obese Patients With Nonalcoholic Fatty Liver DiseasesomayealinaghiNo ratings yet
- Pulse Pressure Is Associated With Alzheimer Biomarkers in Cognitively Normal Older AdultsDocument4 pagesPulse Pressure Is Associated With Alzheimer Biomarkers in Cognitively Normal Older Adultsandreselrey123456No ratings yet
- ALS Seminar Discusses Epidemiology, Genetics, PhenotypesDocument14 pagesALS Seminar Discusses Epidemiology, Genetics, PhenotypesZé CunhaNo ratings yet
- Chromosomal Microarray Versus Karyotyping For Prenatal Diagnosis - 2012Document10 pagesChromosomal Microarray Versus Karyotyping For Prenatal Diagnosis - 2012George CarvalhoNo ratings yet
- Pediatric NAR Trend, Predictors and DifferentialsDocument5 pagesPediatric NAR Trend, Predictors and DifferentialsBariša KiršnerNo ratings yet
- Sex, Gender, and Cardiovascular Health in CanadianDocument8 pagesSex, Gender, and Cardiovascular Health in CanadianLê Huy HoàngNo ratings yet
- ADNIannualchangebiomarkers clinicaloutcomeA&D10Document8 pagesADNIannualchangebiomarkers clinicaloutcomeA&D10brunoabramoffNo ratings yet
- Preoperative Diagnosis of Benign Thyroid Nodules With Indeterminate CytologyDocument11 pagesPreoperative Diagnosis of Benign Thyroid Nodules With Indeterminate CytologyLaraNo ratings yet
- ALS Reversals Demographics Disease Characteristics Treatments and Co MorbiditiesDocument6 pagesALS Reversals Demographics Disease Characteristics Treatments and Co MorbiditiesJoseaneNo ratings yet
- Distribution of ABO Blood Groups and Secretor Status in DiabetesDocument22 pagesDistribution of ABO Blood Groups and Secretor Status in DiabetesSikander GirgoukarNo ratings yet
- Art CarmonaDocument11 pagesArt Carmonarafael martinezNo ratings yet
- Andressa Barreto Glaeser Bruna Lixinski Diniz Desirée Deconte Andressa Schneiders Santos Rafael Fabiano Machado Rosa Paulo Ricardo Gazzola ZenDocument9 pagesAndressa Barreto Glaeser Bruna Lixinski Diniz Desirée Deconte Andressa Schneiders Santos Rafael Fabiano Machado Rosa Paulo Ricardo Gazzola ZenLaura Sanchez SuarezNo ratings yet
- Peters 2014Document10 pagesPeters 2014Andrew E P SunardiNo ratings yet
- Genetic Testing For Inherited Cardiovascular Diseases - A Scientific Statement FRDocument36 pagesGenetic Testing For Inherited Cardiovascular Diseases - A Scientific Statement FRОрися ОбушкоNo ratings yet
- Journal of Rheumatology 2022 - Trajectory of Damage Accrual in SLE Based On Ethnicity and Socioeconomic FactorsDocument7 pagesJournal of Rheumatology 2022 - Trajectory of Damage Accrual in SLE Based On Ethnicity and Socioeconomic FactorsTengku Reza MaulanaNo ratings yet
- Estimating The Prevalence of Dementia and Mild Cognitive Impairment in The US - PMCDocument19 pagesEstimating The Prevalence of Dementia and Mild Cognitive Impairment in The US - PMCveronicalovirgenNo ratings yet
- Management Arteriosclerosis and ClaudicationDocument41 pagesManagement Arteriosclerosis and ClaudicationLuqman AlwiNo ratings yet
- Age at Onset and Parkinson Disease PhenotypeDocument9 pagesAge at Onset and Parkinson Disease Phenotypemariel gonzalezNo ratings yet
- Nej Mo A 1203208Document11 pagesNej Mo A 1203208Iustin TudorNo ratings yet
- 2013 Clinical WES Nejmoa1306555Document10 pages2013 Clinical WES Nejmoa1306555MCuk2606No ratings yet
- Validation of Plasma Amyloid-β 42/40 for Detecting Alzheimer Disease Amyloid PlaquesDocument13 pagesValidation of Plasma Amyloid-β 42/40 for Detecting Alzheimer Disease Amyloid PlaquesHung V. LeNo ratings yet
- ImmunohematologyDocument67 pagesImmunohematologyRainbow SherbetNo ratings yet
- Oligoclonal Bands in Cerebrospinal Fluid of BlackDocument5 pagesOligoclonal Bands in Cerebrospinal Fluid of BlackPaulo Diniz da Gama Instituto do CérebroNo ratings yet
- Sustained Sex-Based Treatment Differences in Acute Coronary Syndrome CareDocument11 pagesSustained Sex-Based Treatment Differences in Acute Coronary Syndrome CarejoNo ratings yet
- J Cardfail 2012 01 017Document8 pagesJ Cardfail 2012 01 017Zi MoonNo ratings yet
- KWF 113Document11 pagesKWF 113Residentes RadiologiaHCENo ratings yet
- Pi Is 0741521414007095Document12 pagesPi Is 0741521414007095Irina NeamtuNo ratings yet
- Prevalence of Diabetic Retinopathy Among Sioux IndiansDocument3 pagesPrevalence of Diabetic Retinopathy Among Sioux IndiansTrx AntraxNo ratings yet
- Preanalytics and Precision PathologyDocument18 pagesPreanalytics and Precision Pathologyedward100% (1)
- Economic and Survival Burden of Dysphagia Among Inpatients in The United StatesDocument7 pagesEconomic and Survival Burden of Dysphagia Among Inpatients in The United StatesClaudia Lorena MedinaNo ratings yet
- Detrano 1989Document7 pagesDetrano 1989Lilims LilithNo ratings yet
- Ni Hms 231483Document7 pagesNi Hms 231483Jose AbadiaNo ratings yet
- Pubmed ElderlyHIV Set.Document24 pagesPubmed ElderlyHIV Set.Ludwig Von MisesNo ratings yet
- A Common Variant of IL-6RDocument27 pagesA Common Variant of IL-6RFarhan Royan PermanahadiNo ratings yet
- Comparación SPEN Pediátrico y AdultoDocument6 pagesComparación SPEN Pediátrico y AdultoVanessa Olate SaavedraNo ratings yet
- Pathogenic Variants in Arteriopathy Genes Detected in A Target 2022 GeneticsDocument11 pagesPathogenic Variants in Arteriopathy Genes Detected in A Target 2022 Geneticsronaldquezada038No ratings yet
- 2023 Peripheral Neuropathy in Older People Is Associated With ReducedDocument8 pages2023 Peripheral Neuropathy in Older People Is Associated With ReducedElisabeth Permatasari SidabutarNo ratings yet
- Caffeine and Risk of Parkinson's Disease in A Large Cohort of Men and WomenDocument7 pagesCaffeine and Risk of Parkinson's Disease in A Large Cohort of Men and WomenJenivia LulileloNo ratings yet
- Circulation: Cardiovascular Quality and OutcomesDocument15 pagesCirculation: Cardiovascular Quality and OutcomesBrent FabialaNo ratings yet
- Metabolic Abnormalities Reveal Chronic Fatigue Syndrome BiologyDocument9 pagesMetabolic Abnormalities Reveal Chronic Fatigue Syndrome BiologyM. A. GARCÍANo ratings yet
- Orginal Healthy Aging Index 2014Document7 pagesOrginal Healthy Aging Index 2014bin linNo ratings yet
- Health Related Quality of Life and Health Insurance Coverage Among - 2017 - BloDocument3 pagesHealth Related Quality of Life and Health Insurance Coverage Among - 2017 - BloMichael John AguilarNo ratings yet
- Clinical Whole-Exome Sequencing For The Diagnosis of Mendelian DisordersDocument10 pagesClinical Whole-Exome Sequencing For The Diagnosis of Mendelian DisordersdssadkjlNo ratings yet
- 10 1016@j Eclinm 2019 05 003Document7 pages10 1016@j Eclinm 2019 05 003Hijaz HijaNo ratings yet
- CERADDocument14 pagesCERADMaría KristjánsdóttirNo ratings yet
- Risk Factors For Mortality in Patients Undergoing Peritoneal Dialysis A Systematic Review and Meta AnalysisDocument12 pagesRisk Factors For Mortality in Patients Undergoing Peritoneal Dialysis A Systematic Review and Meta AnalysisKerstin IbarraNo ratings yet
- Genetic Factors Contributing To Hypertension in African-Based Populations: A Systematic Review and Meta - AnalysisDocument11 pagesGenetic Factors Contributing To Hypertension in African-Based Populations: A Systematic Review and Meta - AnalysisAnonymous K9tmd7T0PzNo ratings yet
- Case Write Up - Dengue FeverDocument22 pagesCase Write Up - Dengue FevervijayaNo ratings yet
- Tujuan DietDocument3 pagesTujuan DietKecengNo ratings yet
- (Cocos Nucifera) The Tree of Life: Coconut in Modern MedicineDocument6 pages(Cocos Nucifera) The Tree of Life: Coconut in Modern MedicineAdharsh ShankaranNo ratings yet
- CPG Gestational Trophoblastic DiseasesDocument31 pagesCPG Gestational Trophoblastic DiseasesSMR50% (2)
- How Environment Affects Our HealthDocument4 pagesHow Environment Affects Our HealthMajo PerezNo ratings yet
- Actividad Unidad 1 Ingles IDocument4 pagesActividad Unidad 1 Ingles Iyohi100% (3)
- Glander DiseaseDocument1 pageGlander DiseaseAdams10No ratings yet
- Continuous Insulin InfusionDocument6 pagesContinuous Insulin Infusionbobobo22No ratings yet
- Redeemer'S Basic School, Yola 3Rd Term Examination Subject: Phe Class: Jss2 Duration: 1 HourDocument3 pagesRedeemer'S Basic School, Yola 3Rd Term Examination Subject: Phe Class: Jss2 Duration: 1 HourRBS RISSNo ratings yet
- National Children's Science Congress: NCSC 2022 and 2023 Focal Theme and Sub-ThemesDocument11 pagesNational Children's Science Congress: NCSC 2022 and 2023 Focal Theme and Sub-ThemesnkhiangteNo ratings yet
- AmJClinNutr 2014 Weaver 1525 42 3Document19 pagesAmJClinNutr 2014 Weaver 1525 42 3Dijana NaloskaNo ratings yet
- Carmelita Divin-Wps OfficeDocument11 pagesCarmelita Divin-Wps OfficeSwen GarciaNo ratings yet
- NIC Claim FormDocument4 pagesNIC Claim FormImtiazNo ratings yet
- Examination Performance Report Spring 2018 Part 1Document3 pagesExamination Performance Report Spring 2018 Part 1Syed Danish AliNo ratings yet
- Qol SonDocument3 pagesQol Sonpermana0adityaNo ratings yet
- Research Proposal On Fast FoodDocument8 pagesResearch Proposal On Fast FoodManish Ravat100% (5)
- Niger DeltaDocument384 pagesNiger DeltaAkinfolami PreferedOne Akinrimisi100% (1)
- BPAC 108 Full TextbookDocument190 pagesBPAC 108 Full Textbook11 AniketNo ratings yet
- Lecture 4 Safety Engineering PracticesDocument40 pagesLecture 4 Safety Engineering PracticesPaolo Manuel NagayoNo ratings yet
- Psychopharmacology Drugs For Disorders - Edited.editedDocument4 pagesPsychopharmacology Drugs For Disorders - Edited.editedMORRIS ANUNDANo ratings yet
- DOH STANDARDS (Indicators) For LEVEL 2 HOSPITALDocument55 pagesDOH STANDARDS (Indicators) For LEVEL 2 HOSPITALMOHBARMM PlanningStaffNo ratings yet
- Shatavari - Benefits, Precautions and DosageDocument13 pagesShatavari - Benefits, Precautions and Dosagenvenkatesh485No ratings yet
- Supported Employment and ModelsDocument19 pagesSupported Employment and ModelsAbhishek Kumar SharmaNo ratings yet
- W9 Teori Akuntansi RS ASPDocument4 pagesW9 Teori Akuntansi RS ASPJessicaNo ratings yet
- Ibm India Benefits - Covid-19 Help Line & Doctor Tele-ConsultationDocument4 pagesIbm India Benefits - Covid-19 Help Line & Doctor Tele-ConsultationSushovan NandiNo ratings yet
- Advance Endoscopy Assessment FormDocument2 pagesAdvance Endoscopy Assessment FormRomelia CampuzanoNo ratings yet
- Functional Requirements For: Pharmacy Information Management SystemsDocument3 pagesFunctional Requirements For: Pharmacy Information Management SystemsShahzeb AmjadNo ratings yet
- Metformin Use in Polycystic Ovary Syndrome (PCOS)Document2 pagesMetformin Use in Polycystic Ovary Syndrome (PCOS)matasatuNo ratings yet
- Prenatal Development and Birth: The Developing Person Through The Life Span Kathleen Stassen Berger - Tenth EditionDocument42 pagesPrenatal Development and Birth: The Developing Person Through The Life Span Kathleen Stassen Berger - Tenth EditionJoel PayneNo ratings yet
- Nephrolithiasis - Drug StudyDocument5 pagesNephrolithiasis - Drug StudyAia JavierNo ratings yet