Professional Documents
Culture Documents
Covalent Bonding and Hybridization 3
Uploaded by
domcruz0308Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Covalent Bonding and Hybridization 3
Uploaded by
domcruz0308Copyright:
Available Formats
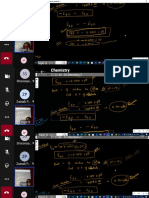
key Points ·
sp +p Ni N N N
I ↓ - I ↓ -
is the 65 : J 65 : J
The hybridization of O or N
2p 2p
23 23
10 :
SpN SpN
same as that of the Cit is JAPN JAPN S ↑PN JAPN
2π : p P -
HS : IP JN
SPN
HS : IP JN
SpN
P P
attached to p P
-
Sp Sp
No sphybridization for oxygen Hybridization of Oxygen
63 : 54546
/
Sp3s p p p : 2 covalent bonds 2 unpaired e
·
+ + +
H H 23 2p
Ex Water
.
H2O sO Is2s22p" 6 Valence electrons S JP IN JNA
As :
bent / angular spe
Geometry tetrahedral
:
sp2s +
p p O C
·
+
sp2H
C 63 : 54546 SY
SY
/
HsN*
AS :
Ex .
Formaldehyde CHO
H
23 2p
spic /PPC
10 :
sp20 sp2S HS :
P JP J
P
1 p P
-
sp2
:
Geometry trigonal planar
:
You might also like
- CHP 6Document8 pagesCHP 6Nada Alseghair0% (1)
- Equations of Motion For The Trebuchet!!!!!!!Document5 pagesEquations of Motion For The Trebuchet!!!!!!!Leo KutsNo ratings yet
- 2 U4 TrigpracticeDocument2 pages2 U4 Trigpracticeapi-292718088No ratings yet
- Attach # 2. Routine Test Reports (Internal) - ReviewedDocument3 pagesAttach # 2. Routine Test Reports (Internal) - ReviewedAvinash PatilNo ratings yet
- Orbital Location of Lone PairsDocument1 pageOrbital Location of Lone Pairsdomcruz0308No ratings yet
- PN Junction Diode I-V & Breakdown CharacteristicsDocument34 pagesPN Junction Diode I-V & Breakdown CharacteristicsVikas VarshneyNo ratings yet
- Covalent Bonding and Hybridization 2Document1 pageCovalent Bonding and Hybridization 2domcruz0308No ratings yet
- Ooeebe: HybridizaronDocument4 pagesOoeebe: HybridizaronKira BezkorovainaNo ratings yet
- Summary of Institute Timing - Students - SOEDocument1 pageSummary of Institute Timing - Students - SOESHAH HARSHILNo ratings yet
- บทที่ 1Document2 pagesบทที่ 1s6604052616034No ratings yet
- Lorenzo Donati - Sicut Cervus - For 16 VoicesDocument9 pagesLorenzo Donati - Sicut Cervus - For 16 VoicesLorenzo DonatiNo ratings yet
- Plane SplittingDocument1 pagePlane SplittingPie CrustNo ratings yet
- Shell: Enters SubDocument22 pagesShell: Enters SubKashish SinghNo ratings yet
- Attendence Sheet: Students DetailsDocument3 pagesAttendence Sheet: Students DetailsmuditNo ratings yet
- 31p NMRDocument17 pages31p NMRperulageaNo ratings yet
- Scriabin Prelude Op11no1-A4Document1 pageScriabin Prelude Op11no1-A4franky_dNo ratings yet
- Introduction To PhosphoproteomicsDocument14 pagesIntroduction To PhosphoproteomicsbiolabpartnerNo ratings yet
- สมุดโน้ต PDFDocument3 pagesสมุดโน้ต PDFsssssNo ratings yet
- STA 342-TH7-Test On Equality of Population ProportionsDocument5 pagesSTA 342-TH7-Test On Equality of Population Proportionssolomon mwatiNo ratings yet
- Adobe Scan 24-May-2022Document4 pagesAdobe Scan 24-May-2022PupunNo ratings yet
- Test EDocument1 pageTest EDavid AppelsNo ratings yet
- Transfer Cases PDFDocument1 pageTransfer Cases PDFpvaibhyNo ratings yet
- List of Students Transferred To Group 2Document1 pageList of Students Transferred To Group 2pvaibhyNo ratings yet
- 2.MSA 计数型Document22 pages2.MSA 计数型cong daNo ratings yet
- Hyperbola PropertiesDocument4 pagesHyperbola PropertiesArsh DhawanNo ratings yet
- Insulin Receptor PathwayDocument1 pageInsulin Receptor PathwayAlexe VillagomezNo ratings yet
- #Lecture 3 - Cam Clay Undrained CompDocument12 pages#Lecture 3 - Cam Clay Undrained CompWanNo ratings yet
- LaberintoDocument1 pageLaberintoChacha BinxNo ratings yet
- TRPV6 Pip2Document37 pagesTRPV6 Pip2Xing-Zhen ChenNo ratings yet
- PharmacophorePatterns PDFDocument29 pagesPharmacophorePatterns PDFIoana Mirela VasincuNo ratings yet
- Proteins Section TutorialDocument2 pagesProteins Section TutorialPapama KwayimaniNo ratings yet
- Tally SheetDocument2 pagesTally SheetReyzalyn LagrimasNo ratings yet
- Ground Floor Plan Lighting LayoutDocument1 pageGround Floor Plan Lighting LayoutALLEN MAACNo ratings yet
- TP16 Copia 2 (Contrapunto) FINAL DE CONTRAPUTODocument1 pageTP16 Copia 2 (Contrapunto) FINAL DE CONTRAPUTOMaca GarciaNo ratings yet
- Ethane: H HybridizationDocument37 pagesEthane: H HybridizationCatalinaNo ratings yet
- A Man'S Destiny: 'Yn Ieren en Sinen'Document14 pagesA Man'S Destiny: 'Yn Ieren en Sinen'Theseus EditionNo ratings yet
- Emeng 3131 Electrical Power Systems: Power System Transients, Power System Stability & Load Flow StudiesDocument40 pagesEmeng 3131 Electrical Power Systems: Power System Transients, Power System Stability & Load Flow StudiesmichaelNo ratings yet
- HW 3Document2 pagesHW 3Arnold KeNo ratings yet
- Proizvodnja Praskastih - DeterdzenataDocument6 pagesProizvodnja Praskastih - DeterdzenataDragon's Sin of Wrath MeliodasNo ratings yet
- Lamera L-Section PDFDocument8 pagesLamera L-Section PDFramthakurh12No ratings yet
- Plan A1 A4Document4 pagesPlan A1 A4Joebany SaboteNo ratings yet
- Foundation Rev.01Document1 pageFoundation Rev.01sopheayem168No ratings yet
- Bourree em BWV996Document1 pageBourree em BWV996c1-1zNo ratings yet
- Bourree A4 PDFDocument1 pageBourree A4 PDFaguilarjmNo ratings yet
- Bourrée in E Minor: Lute or Lute-Harpischord (Lautenwerk)Document1 pageBourrée in E Minor: Lute or Lute-Harpischord (Lautenwerk)Grado ZeroNo ratings yet
- Piano Bourree in E Minor PDFDocument1 pagePiano Bourree in E Minor PDFFelipe Velez100% (1)
- Bourrée in E Minor: Lute or Lute-Harpischord (Lautenwerk)Document1 pageBourrée in E Minor: Lute or Lute-Harpischord (Lautenwerk)Ariel PuertaNo ratings yet
- 1 Slutsky Matrix Og Negative Deniteness: 1.1 SolutionDocument5 pages1 Slutsky Matrix Og Negative Deniteness: 1.1 SolutionDeepali PatilNo ratings yet
- Underground (Symphony Sessions) - ScoreDocument25 pagesUnderground (Symphony Sessions) - ScoreAnonymous dQi2IXNo ratings yet
- Symphony X - Evolution The Grand Design (Solo)Document7 pagesSymphony X - Evolution The Grand Design (Solo)Franco SeminarioNo ratings yet
- Lecture 15Document20 pagesLecture 15Yavuz KaplanNo ratings yet
- Metamorphosis Orchestral Sketches - Horn in F 2Document1 pageMetamorphosis Orchestral Sketches - Horn in F 2Amir Sanjary ComposerNo ratings yet
- D2 PDFDocument16 pagesD2 PDFAnonymous TThmYKFpNo ratings yet
- II PUC PHYSICS - Previously Appeared Questions, Important Questions and Answers For 2023 Exam by MANJUNATH B.Document53 pagesII PUC PHYSICS - Previously Appeared Questions, Important Questions and Answers For 2023 Exam by MANJUNATH B.praveens.photos.2023No ratings yet
- Attendance Sheet For The Month May, 17 From 21 Apr To 20 MayDocument3 pagesAttendance Sheet For The Month May, 17 From 21 Apr To 20 MayShabirNo ratings yet
- Clones 4m1Document26 pagesClones 4m1keman91No ratings yet
- BUKU - RETRIBUSI - HARIAN - September 2020Document44 pagesBUKU - RETRIBUSI - HARIAN - September 2020Ab Daaddann DHauriNo ratings yet
- The Promise Concert Score PDFDocument8 pagesThe Promise Concert Score PDFmakmare93No ratings yet
- Etherna Wait For No Answer - Baritone SaxophoneDocument4 pagesEtherna Wait For No Answer - Baritone SaxophonePanosNo ratings yet
- 2017-Thermal Compression Bonding Understanding Heat Transfer by in Situ Measurements and ModelingDocument7 pages2017-Thermal Compression Bonding Understanding Heat Transfer by in Situ Measurements and ModelingDanNo ratings yet
- Chapter 2 How Time and Interest Affect MoneyDocument22 pagesChapter 2 How Time and Interest Affect MoneyFlorDelaRamaNo ratings yet
- Final Sample Paper Booklet 2021-22Document84 pagesFinal Sample Paper Booklet 2021-22Nitin SinghNo ratings yet
- Molecular Orbitals in Hetero Nuclear Diatomic Molecules: FOR I M.SC A& B VICAS StudentsDocument10 pagesMolecular Orbitals in Hetero Nuclear Diatomic Molecules: FOR I M.SC A& B VICAS StudentsJeevanantham VelayuthamNo ratings yet
- Din en 10130Document14 pagesDin en 10130Ricardo VitorianoNo ratings yet
- Chapter 5 - Bu2Document4 pagesChapter 5 - Bu2mjcntn000No ratings yet
- Sintering Performance of Magnetite-Hematite-GoethiDocument19 pagesSintering Performance of Magnetite-Hematite-GoethiDeidaNo ratings yet
- Ch6 LateralEarthPressureDocument39 pagesCh6 LateralEarthPressure彭宇鑫No ratings yet
- Densit® Wear Protection ProductsDocument9 pagesDensit® Wear Protection ProductsMuhammad IqbalNo ratings yet
- Aging Characteristics of RTV Silicone Rubber Insulator CoatingsDocument9 pagesAging Characteristics of RTV Silicone Rubber Insulator Coatingskhanh khanhNo ratings yet
- Astm E309-01Document5 pagesAstm E309-01Carlos Raul Caballero LeonNo ratings yet
- Environmental Science Lesson 1-2Document6 pagesEnvironmental Science Lesson 1-2Paolo jay LoristoNo ratings yet
- Gas ChromatographyDocument12 pagesGas ChromatographyYaman Adnan QabajaNo ratings yet
- Ladle NozzleDocument6 pagesLadle Nozzlejagd.shresthaNo ratings yet
- TFX02 Torque ChartDocument1 pageTFX02 Torque ChartArnulfo SánchezNo ratings yet
- 05 - SPSF1 07 B5Document14 pages05 - SPSF1 07 B5Anonymous RfKC0LciBK0% (1)
- Type Series Booklet: Submersible Borehole PumpDocument104 pagesType Series Booklet: Submersible Borehole PumpJoao PauloNo ratings yet
- ETAP Workshop Notes ANSI Short Circuit Example: DescriptionDocument6 pagesETAP Workshop Notes ANSI Short Circuit Example: DescriptionGanti KameshNo ratings yet
- Structure Plasma Membrane: of TheDocument4 pagesStructure Plasma Membrane: of TheYaserhaidar AlgotaryNo ratings yet
- Lesson2 1-LightDocument4 pagesLesson2 1-LightGrace06 LabinNo ratings yet
- LV Cable SLP-BH-1 To Sa2-011Document4 pagesLV Cable SLP-BH-1 To Sa2-011Vijayan Thekke VeeduNo ratings yet
- Topic 2 - MechanicsDocument104 pagesTopic 2 - MechanicsJunting HuNo ratings yet
- 5 Spheres and ConesDocument8 pages5 Spheres and Conesstemtutor kakwongNo ratings yet
- MODULE 3 Unit 1 Behavior of DC Shunt, Compound and Series MotorsDocument30 pagesMODULE 3 Unit 1 Behavior of DC Shunt, Compound and Series MotorsJister EstrasNo ratings yet
- Jee (M+a) It 1 Nurture PH 1 03 May 23 (2023 24) NoticeDocument3 pagesJee (M+a) It 1 Nurture PH 1 03 May 23 (2023 24) NoticeARAVIND SANTHOSHNo ratings yet
- SECTION 23 65 00 Cooling TowersDocument28 pagesSECTION 23 65 00 Cooling TowersDivyansh Singh ChauhanNo ratings yet
- Kafuter K 704Document2 pagesKafuter K 704ketab_doostNo ratings yet