Professional Documents
Culture Documents
Percentage of Oxygen
Uploaded by
mahdee khanCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Percentage of Oxygen
Uploaded by
mahdee khanCopyright:
Available Formats
Page |1
NAME Mahdee Khan ID 85551 TIME 15 Mins TOTAL MARKS 15
Topic: Percentage of Oxygen
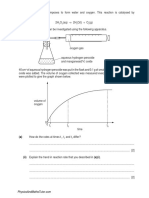
1. A teacher uses this apparatus to find the percentage of oxygen in a gaseous mixture of oxygen and argon.
This is the teacher’s method.
Step 1 heat the copper powder
Step 2 push the plunger on syringe A to pass the mixture of oxygen and argon over the hot copper so that the mixture
moves into syringe B
Step 3 push the plunger on syringe B to pass the mixture of oxygen and argon over the hot copper so that the mixture
moves into syringe A
Step 4 record the reading on syringe A
Step 5 repeat Steps 2, 3 and 4 a number of times
The volume of gas decreases as the oxygen reacts with the copper. Argon is unreactive so does not react with the copper. The
copper powder turns black.
(a) (i) Give a reason why the copper powder is heated. (1)
....................................................................................................................................................................................
(ii) State why argon is unreactive. (1)
....................................................................................................................................................................................
(iii) Give the name of the black powder that forms when the oxygen reacts with the copper. (1)
...................................................................................................................................................................................
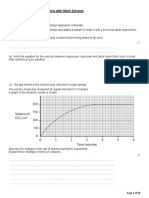
(b) The table shows the teacher’s results.
Reading number Start 1 2 3 4 5 6 7 8 9 10
Reading syringe A in Cm3 78 70 67 65 63 61 60 59 58 58 58
(i) State how the results show that all the oxygen has reacted. (1)
....................................................................................................................................................................................
(ii) The volume of gas in the glass tube and connecting tubes is 175 cm 3. Use this value and the results table to calculate the
percentage of oxygen in the mixture of oxygen and argon. (3)
percentage of oxygen = .............................................................. %
Md. Mamun Ur Rashid, Chemistry Teacher, Cell: 01853605525, 01675240922
Page |2
(iii) Suggest one reason why the calculated percentage of oxygen in the mixture may not be accurate. (1)
....................................................................................................................................................................................
(Total for Question 1 = 8 marks)
2. This question is about rusting.
(a) When iron rusts, it reacts with oxygen in the air.
A student uses the rusting of iron to find the percentage of oxygen in a sample of air. The diagram shows the apparatus.
These are the student’s results.
volume of air in conical flask and connecting tube = 265 cm 3
volume of air in gas syringe at start = 100 cm3
volume of air in gas syringe at end = 25 cm3
Calculate the percentage of oxygen in the sample of air using the student’s results. (3)
percentage of oxygen = .............................................................. %
(b) (i) Cars are painted to prevent the iron in car bodies from rusting. Explain how painting prevents the iron in car bodies from
rusting. (2)
.....................................................................................................................................................................................
.....................................................................................................................................................................................
(ii) Some car manufacturers use paint containing tiny particles of zinc.
Explain how particles of zinc prevent iron in car bodies from rusting even when this paint is scratched. (2)
....................................................................................................................................................................................
...................................................................................................................................................................................
(Total for Question 2 = 7 Marks)
Md. Mamun Ur Rashid, Chemistry Teacher, Cell: 01853605525, 01675240922
You might also like
- Amount of Substance 1 QPDocument10 pagesAmount of Substance 1 QPHajhoj CellNo ratings yet
- As-Level Paper 1 pp9Document15 pagesAs-Level Paper 1 pp9ConorNo ratings yet
- Separate Chemistry: Higher Tier in BoldDocument16 pagesSeparate Chemistry: Higher Tier in BoldzipperNo ratings yet
- Read These Instructions First: 7 Printed PagesDocument7 pagesRead These Instructions First: 7 Printed PagesZainab ShigriNo ratings yet
- Calculations QDocument67 pagesCalculations Q13684962573No ratings yet
- Energetics Exam QsDocument5 pagesEnergetics Exam Qszadinova.tereza16No ratings yet
- Topic 9 Past Papers Questions With Mark SchemeDocument60 pagesTopic 9 Past Papers Questions With Mark SchemeQasim PerachaNo ratings yet
- Haberprocess 1Document65 pagesHaberprocess 1AbdulBasitBilalSheikhNo ratings yet
- Chemistry c1 Core PracticalsDocument18 pagesChemistry c1 Core PracticalsgriggansNo ratings yet
- Chemical Equilibrium: A2 Chemistry (Unit 4)Document9 pagesChemical Equilibrium: A2 Chemistry (Unit 4)Maliha Ishrat JarinNo ratings yet
- As Level Chemistry: Topic 2 - Amount of Substance TestDocument10 pagesAs Level Chemistry: Topic 2 - Amount of Substance Testkarima akterNo ratings yet
- The Ideal Gas Equation 7 QPDocument5 pagesThe Ideal Gas Equation 7 QPjade.davis0019No ratings yet
- Cons. of Mass, Chemical Measurements - Eqns 2 QPDocument18 pagesCons. of Mass, Chemical Measurements - Eqns 2 QPHusain KamaliNo ratings yet
- 9701 m16 QP 52Document8 pages9701 m16 QP 52SanthiKalyanaGrantNo ratings yet
- Chemistry End of Year IncompleteDocument5 pagesChemistry End of Year IncompleteSebastian ZhangNo ratings yet
- Oct Mock RevisionDocument16 pagesOct Mock RevisionAdamNo ratings yet
- Measurements and ErrorsDocument89 pagesMeasurements and Errorselsiesaveena96No ratings yet
- Nitroglycerine, C3H5N3O9, Is An Explosive Which, On Detonation, Decomposes RapidlyDocument14 pagesNitroglycerine, C3H5N3O9, Is An Explosive Which, On Detonation, Decomposes Rapidlyapi-25909541No ratings yet
- Chemistry Alternative To PracticalDocument4 pagesChemistry Alternative To PracticalMCHNo ratings yet
- As-Level Paper 1 pp1Document16 pagesAs-Level Paper 1 pp1Rivanti Savira PramuditaNo ratings yet
- 1.structure Bonding N Intro To Organic Bonding QuestionsDocument44 pages1.structure Bonding N Intro To Organic Bonding QuestionskamrunnisaNo ratings yet
- A-Level Chemistry: Paper 3 Practice Paper 3Document20 pagesA-Level Chemistry: Paper 3 Practice Paper 3Jesus ChristNo ratings yet
- Work Book Rate of Reaction ATP+ Practical Grade 9 ErumDocument20 pagesWork Book Rate of Reaction ATP+ Practical Grade 9 ErumJavariaAjmalNo ratings yet
- Revision Questions Unit 1Document73 pagesRevision Questions Unit 1manoleionescumaNo ratings yet
- 1.4 TestDocument5 pages1.4 TestdfghjNo ratings yet
- Edexcel IGCSE May 2012 Chemistry Paper - 2Document16 pagesEdexcel IGCSE May 2012 Chemistry Paper - 2Coolman PoonNo ratings yet
- BondingDocument14 pagesBondingMuizzudin AzaliNo ratings yet
- Ammonia Revision QuestionsDocument64 pagesAmmonia Revision QuestionsfikaduNo ratings yet
- Water and HydrogenDocument7 pagesWater and HydrogenOkumu KevinsNo ratings yet
- Organic Anaylsis: Aqa As ChemistryDocument17 pagesOrganic Anaylsis: Aqa As ChemistryHamza AbulailaNo ratings yet
- Moles Calculation Paper A 2023 Yr 11Document11 pagesMoles Calculation Paper A 2023 Yr 11Hi :DNo ratings yet
- Group 7Document59 pagesGroup 7nw4dkcn6vwNo ratings yet
- Year 10 EOT3 Revison Booklet With AnswerDocument99 pagesYear 10 EOT3 Revison Booklet With AnswerAdamNo ratings yet
- Practice Questions - Biology - Year 10Document24 pagesPractice Questions - Biology - Year 10thomasNo ratings yet
- Chem Mock Test IG A Paper I (2023)Document18 pagesChem Mock Test IG A Paper I (2023)pyae157163No ratings yet
- Respiration - Questions PDFDocument25 pagesRespiration - Questions PDFHolly Robinson0% (1)
- Rates-Of-Reaction Questions IGCSEDocument8 pagesRates-Of-Reaction Questions IGCSEToni ANo ratings yet
- Paper 3 Practice Paper 4Document22 pagesPaper 3 Practice Paper 4EmoryNo ratings yet
- Cambridge International General Certifi Cate of Secondary EducationDocument12 pagesCambridge International General Certifi Cate of Secondary EducationDark GreenNo ratings yet
- Rate of Reaction 2 QP (Tomek)Document9 pagesRate of Reaction 2 QP (Tomek)Tomasz OstrowskiNo ratings yet
- Limiting Factors 6Document7 pagesLimiting Factors 6Khadija AhmedNo ratings yet
- Bonding - Structuce 3 QPDocument14 pagesBonding - Structuce 3 QPtertianerima101No ratings yet
- Changestotheatmosphere 2Document74 pagesChangestotheatmosphere 2Eyad MohamedNo ratings yet
- AQA C2 Past Paper Q&A Part 1Document184 pagesAQA C2 Past Paper Q&A Part 1Junaid AsgharNo ratings yet
- Isomerism 2 QPDocument9 pagesIsomerism 2 QPPragna AnanthNo ratings yet
- Quiz 1Document23 pagesQuiz 1FIKRIYE ONDEROLNo ratings yet
- 1.3 Atoms Molecules Stoichiometry Theory Ial Cie Chemistry QP UnlockedDocument10 pages1.3 Atoms Molecules Stoichiometry Theory Ial Cie Chemistry QP UnlockedArawole ToyosiNo ratings yet
- Plants Questions ks3Document4 pagesPlants Questions ks3Eshal AmirNo ratings yet
- Radioactivity and ParticlesDocument10 pagesRadioactivity and ParticlesASMNo ratings yet
- Titration Calculations 1Document55 pagesTitration Calculations 1Segun HursheeNo ratings yet
- 1 Hydrogen Peroxide Decomposes To Form Water and Oxygen. This Reaction Is Catalysed byDocument9 pages1 Hydrogen Peroxide Decomposes To Form Water and Oxygen. This Reaction Is Catalysed byKelvin DenhereNo ratings yet
- MockDocument13 pagesMockhozayenmaiNo ratings yet
- Review IB ChemistryDocument18 pagesReview IB ChemistryAlshaimaa SolimanNo ratings yet
- Cambridge IGCSE: CHEMISTRY 0620/42Document16 pagesCambridge IGCSE: CHEMISTRY 0620/42Manya PunjabiNo ratings yet
- Organics QuestionsDocument3 pagesOrganics Questionsnairamathrawala3000No ratings yet
- Mixed Topic Revision 1 DiffusionDocument23 pagesMixed Topic Revision 1 DiffusionYaakkwNo ratings yet
- Eco and Envir 2019 Sep SciDocument13 pagesEco and Envir 2019 Sep SciStudent Valeria Ferriol CebrianNo ratings yet
- Advanced Synthesis of Gold and Zirconia Nanoparticles and their CharacterizationFrom EverandAdvanced Synthesis of Gold and Zirconia Nanoparticles and their CharacterizationNo ratings yet
- BeamDocument18 pagesBeamAfia S Hameed100% (1)
- 2BH1900 Ie2 122010 enDocument2 pages2BH1900 Ie2 122010 enChan Chi Wong PenNo ratings yet
- CRS, TIRUPATI FINAL ESTIMATE FinalDocument637 pagesCRS, TIRUPATI FINAL ESTIMATE FinalSatish PatnalaNo ratings yet
- CH - 005 Supervision of Pile DrivingDocument3 pagesCH - 005 Supervision of Pile DrivingCza ManNo ratings yet
- Mineral India Cement 2013Document10 pagesMineral India Cement 2013Vipin MohanNo ratings yet
- Thermodynamics FinalDocument26 pagesThermodynamics FinalgautamahujaNo ratings yet
- NZS 1664-1-1997 Aluminium Structures Limit State DesignDocument8 pagesNZS 1664-1-1997 Aluminium Structures Limit State Designwey53160% (1)
- Arctica Engine KavoDocument14 pagesArctica Engine KavoHsuan ChenNo ratings yet
- (Jens J. Ernberg, Stanley E. Asnis (Auth.), Stanle (B-Ok - CC) PDFDocument337 pages(Jens J. Ernberg, Stanley E. Asnis (Auth.), Stanle (B-Ok - CC) PDFEnquiry SgNo ratings yet
- TEC - TA-550 - Power Grout Spec SheetDocument2 pagesTEC - TA-550 - Power Grout Spec SheetMark MehnerNo ratings yet
- Nir 9 GB LevelDocument5 pagesNir 9 GB LevelYuri OrellanoNo ratings yet
- MSS SP-117 (1996) PDFDocument11 pagesMSS SP-117 (1996) PDFadprim100% (1)
- Manual UT Thickness Specific Exam From SAEP-1146 Name: Badge#: DateDocument5 pagesManual UT Thickness Specific Exam From SAEP-1146 Name: Badge#: DateMohamed IbrahimNo ratings yet
- Three Span Continuos Straight Coposite I GirderDocument32 pagesThree Span Continuos Straight Coposite I GirderMarlon MartinezNo ratings yet
- CE 1999 GATE Question PaperDocument14 pagesCE 1999 GATE Question Papersubhajit284No ratings yet
- Huskie CatalogDocument104 pagesHuskie Catalogsf94y5t2ryNo ratings yet
- Blowdown System Boiler BookDocument16 pagesBlowdown System Boiler BookMatias MancillaNo ratings yet
- NX Mold DesignDocument3 pagesNX Mold DesignNguyễn Thế Quang DũngNo ratings yet
- When Some: Tlie Tlie Is It IsDocument1 pageWhen Some: Tlie Tlie Is It IsreacharunkNo ratings yet
- Bill of MaterialsDocument2 pagesBill of MaterialsJonathan SilayaNo ratings yet
- Reducerea FrecariiDocument82 pagesReducerea FrecariiDragomir IsabellaNo ratings yet
- Interactive Schematic: This Document Is Best Viewed at A Screen Resolution of 1024 X 768Document25 pagesInteractive Schematic: This Document Is Best Viewed at A Screen Resolution of 1024 X 768juan echeverryNo ratings yet
- Determination of Boiling Point of Organic CompoundsDocument28 pagesDetermination of Boiling Point of Organic CompoundsDotsha Raheem83% (6)
- Ppce Unit - I Process Planning and Cost EstimationDocument14 pagesPpce Unit - I Process Planning and Cost EstimationJacob RubasinghNo ratings yet
- Wsc2015 Tp10 PV TW Assembly Iso A PreDocument1 pageWsc2015 Tp10 PV TW Assembly Iso A PreCristyan ReisNo ratings yet
- Nagoya Institute of TechnologyDocument13 pagesNagoya Institute of TechnologyOnline EarningNo ratings yet
- AISC Seismic Design-ModuleUG-Brief OverviewDocument86 pagesAISC Seismic Design-ModuleUG-Brief OverviewDemçe Florjan100% (1)
- Astm 638MDocument12 pagesAstm 638Mjose diazNo ratings yet
- #Changes in HSN Code - Custom Tarif Act - 01.05.2023 PDFDocument2 pages#Changes in HSN Code - Custom Tarif Act - 01.05.2023 PDFSephali AbhangiNo ratings yet
- Project Standard Specification: Fuel Gas Piping 15194 - Page 1/17Document17 pagesProject Standard Specification: Fuel Gas Piping 15194 - Page 1/17adel rihanaNo ratings yet