Professional Documents
Culture Documents
Problem Session Remi
Uploaded by
Student BpharmaCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Problem Session Remi
Uploaded by
Student BpharmaCopyright:
Available Formats
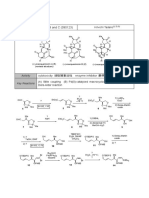
1. Please provide a plausible mechanism for the following transformation, explaining the complete diastereoselectivity obtained. (Org. Lett.
2020, 22, 21, 8555)
HO
NH2 HN
O Dy(OTf)3
+
DCE, 90 °C O
N
Me N
Me
70% yield
2. Please provide a plausible mechanism for the following transformation. (Org. Lett. 2021, 23, 20, 7771–7775)
OTMS 1) TsN3, CuTc, PhMe
CHO 2) Rh2(OCt)4 (2 mol%), PhMe, 90 °C OTMS
then Al2O3
OTMS O
TMSO
CHO
64% yield
3. Please provide a plausible mechanism for the following transformation. (J. Am. Chem. Soc. 2021, 143, 21270−21274)
OH
NHBoc PdCl2, P(4-F-C6H4)3 O O
O NBE, Cs2CO3, MeCN
then HCl, Fe2(SO4)3
O O
+ O
I (4 steps)
OH
NBE: HO
Br
O
65% yield Dalesconol A
4. Please provide a plausible mechanism for the following transformations and the structure of the isolable intermediate. (Org. Lett. 2016, 18, 3952−3955)
EtO2C
O 1) ethyl diazoacetate
LDA, THF, - 78 °C CsF
OTMS 2) In(OTf)3, 4 A MS, CH2Cl2 N
Boc H
N TMS MeCN, reflux
66% yield 60% yield
(2 steps)
You might also like
- Chemical EOR S2 1Document199 pagesChemical EOR S2 1Amry Sitompul100% (1)
- Fukuyama Group - Group Meeting Problems 07/04/2017Document4 pagesFukuyama Group - Group Meeting Problems 07/04/2017Huỳnh ĐặngNo ratings yet
- Catalytic Asymmetric Total Synthesis of Exiguolide: Chem. Eur. J. 2020, Accepted Manuscript Doi:10.1002/chem.202001773Document2 pagesCatalytic Asymmetric Total Synthesis of Exiguolide: Chem. Eur. J. 2020, Accepted Manuscript Doi:10.1002/chem.202001773Chem MistryNo ratings yet
- 3 PDocument3 pages3 PHồ Đức ViệtNo ratings yet
- Fukuyama Group - Group Meeting Problems 2001/08/22: N N N HDocument2,429 pagesFukuyama Group - Group Meeting Problems 2001/08/22: N N N HGia PhướcNo ratings yet
- 2018 PDFDocument32 pages2018 PDFDicky Tak Hin WongNo ratings yet
- Adv Retrosynthesis PDFDocument29 pagesAdv Retrosynthesis PDFericaNo ratings yet
- Group Meeting Problems 2021/12/11: Co Me N N Meo C Co Me Meo C CHCL 80 (R BN) Meoh, RT (2 Eq)Document5 pagesGroup Meeting Problems 2021/12/11: Co Me N N Meo C Co Me Meo C CHCL 80 (R BN) Meoh, RT (2 Eq)dicky wongNo ratings yet
- Group Meeting Problems 2021/10/02Document9 pagesGroup Meeting Problems 2021/10/02dicky wongNo ratings yet
- Kaitocephalin-2 - USDocument2 pagesKaitocephalin-2 - USPercival GalahadNo ratings yet
- Practice Problems On Carboxylic Acid DerivativesDocument3 pagesPractice Problems On Carboxylic Acid DerivativesNeil GaymanNo ratings yet
- Synthesis of Cyclic and Acyclic B-Amino Acids Via Chelation-Controlled 1,3-Dipolar CycloadditionDocument16 pagesSynthesis of Cyclic and Acyclic B-Amino Acids Via Chelation-Controlled 1,3-Dipolar CycloadditionNguyễn Thái DươngNo ratings yet
- Letitia Biweeklyreport 2Document5 pagesLetitia Biweeklyreport 2Anonymous hCWbXhgNo ratings yet
- Cum 130420Document1 pageCum 130420Huỳnh ĐặngNo ratings yet
- Ethyl 4 - (Triphenylphosphoranylidene) - Acetoacetate: O Tro Oh O Tro Co Et ODocument4 pagesEthyl 4 - (Triphenylphosphoranylidene) - Acetoacetate: O Tro Oh O Tro Co Et OPhạm Gia KhánhNo ratings yet
- Aryne ProblemsDocument1 pageAryne Problems21 01 15 Tường LâmNo ratings yet
- 1992 Tetrahedron Kolb Stereospecific 1 - 2 DiolsDocument16 pages1992 Tetrahedron Kolb Stereospecific 1 - 2 Diolsjames mellaleievNo ratings yet
- Short Answer Questions: Chem223 Practice Exam (Pre Final)Document2 pagesShort Answer Questions: Chem223 Practice Exam (Pre Final)Jenny WangNo ratings yet
- Adv. Mater. 2020 (32) 1906128 SIDocument48 pagesAdv. Mater. 2020 (32) 1906128 SIMelgious AngNo ratings yet
- Aromatic Problems 2013Document4 pagesAromatic Problems 2013YocobSamandrewsNo ratings yet
- Total Synthesis of ( ) - Sinulariadiolide. A Transannular ApproachDocument2 pagesTotal Synthesis of ( ) - Sinulariadiolide. A Transannular ApproachChem MistryNo ratings yet
- (+) - Macquarimicins A, B and C (090123) : Kin-Ichi TadanoDocument3 pages(+) - Macquarimicins A, B and C (090123) : Kin-Ichi TadanoPercival GalahadNo ratings yet
- Ioc 11Document5 pagesIoc 11KarthikeyanNo ratings yet
- Imperial College LondonDocument1 pageImperial College LondonCalum GlynnNo ratings yet
- Activity: O Me Me BR H OHDocument2 pagesActivity: O Me Me BR H OHPercival GalahadNo ratings yet
- Selectivity in Organic SynthesiDocument5 pagesSelectivity in Organic SynthesiChris LittleNo ratings yet
- Asymmetric Aldol/Vinylogous Aldol Reaction Catalyzed by Chiral Phosphine Oxide: Stereoselective Synthesis of 4-PyranonesDocument1 pageAsymmetric Aldol/Vinylogous Aldol Reaction Catalyzed by Chiral Phosphine Oxide: Stereoselective Synthesis of 4-PyranonesNathan Ray AlimNo ratings yet
- Activity: O H OH OH OH OH O HDocument3 pagesActivity: O H OH OH OH OH O HPercival GalahadNo ratings yet
- Pestalotiopsin A - USDocument2 pagesPestalotiopsin A - USPercival GalahadNo ratings yet
- Asymmetric Total Synthesis of Mycoleptodiscin A: Angew. Chem. Int. Ed. 2015, 54, 6878-6882Document2 pagesAsymmetric Total Synthesis of Mycoleptodiscin A: Angew. Chem. Int. Ed. 2015, 54, 6878-6882Chem MistryNo ratings yet
- Towards A First-Principles Chemical Engineering: Karsten ReuterDocument28 pagesTowards A First-Principles Chemical Engineering: Karsten ReuterMahfuzur Rahman SiddikyNo ratings yet
- Group Meeting Problems 2022/05/07: O OCH H Cyclohexane, RT 90% O OOHDocument6 pagesGroup Meeting Problems 2022/05/07: O OCH H Cyclohexane, RT 90% O OOHdicky wongNo ratings yet
- Chem 1140 Ring-Closing Metathesis (RCM) and Ring-Opening Metathesis (ROMP)Document26 pagesChem 1140 Ring-Closing Metathesis (RCM) and Ring-Opening Metathesis (ROMP)Abhishek GuddadNo ratings yet
- Final Sim CorrDocument12 pagesFinal Sim CorrKeila BoisjolyNo ratings yet
- Journal Pre-Proofs: Bioorganic & Medicinal ChemistryDocument18 pagesJournal Pre-Proofs: Bioorganic & Medicinal ChemistryWalid Ebid ElgammalNo ratings yet
- (+) - Lasonolide A (120414-TKGP) K. Shishido: ActivityDocument3 pages(+) - Lasonolide A (120414-TKGP) K. Shishido: ActivityPercival GalahadNo ratings yet
- Strategy in Synthesis-08Document34 pagesStrategy in Synthesis-08fjewafhjeashfeshfNo ratings yet
- PracticeTests Answers All Chem360Document109 pagesPracticeTests Answers All Chem360EthanNo ratings yet
- Central Core Uprolides A Survey of Some Ring Closing Metathesis ApproachesDocument6 pagesCentral Core Uprolides A Survey of Some Ring Closing Metathesis Approachessunaina agarwalNo ratings yet
- Problems Seminar 2 - 2023 - 231004 - 144845Document7 pagesProblems Seminar 2 - 2023 - 231004 - 144845hectormunozroNo ratings yet
- Co So Ly Thuyet Hoa Huu Co - Bao HayDocument14 pagesCo So Ly Thuyet Hoa Huu Co - Bao HayGia Huy DuongNo ratings yet
- Synthesis Challenge #5 AG Wegner Total Synthesis of (-) - Nakadomarin ADocument5 pagesSynthesis Challenge #5 AG Wegner Total Synthesis of (-) - Nakadomarin ADuc Thien NguyenNo ratings yet
- Synthesis of NitrochalconesDocument8 pagesSynthesis of NitrochalconesKassimNo ratings yet
- Chrommatography Introduction-Handout-2023Document49 pagesChrommatography Introduction-Handout-2023Hien HoangNo ratings yet
- Pract Prob Carboxylic Acids AnsDocument3 pagesPract Prob Carboxylic Acids AnsVictor HernandezNo ratings yet
- Chemical Kinetics of Combustion Processes: University of Southern CaliforniaDocument18 pagesChemical Kinetics of Combustion Processes: University of Southern CaliforniaMadson John ArcanjoNo ratings yet
- Org. Synth. 2005, 81, 33-41 (Fer Discussion)Document15 pagesOrg. Synth. 2005, 81, 33-41 (Fer Discussion)ludoNo ratings yet
- ICh O2022Document15 pagesICh O2022Monica IonitaNo ratings yet
- Group Meeting Problems 2023/03/11: Oh Nhboc O O O O Oh Otbs O O O O O O Cpru (PPH) CL, PPH NPF NahcoDocument1 pageGroup Meeting Problems 2023/03/11: Oh Nhboc O O O O Oh Otbs O O O O O O Cpru (PPH) CL, PPH NPF NahcoroccenxuNo ratings yet
- Electronic Supplementary Information (ESI) ForDocument8 pagesElectronic Supplementary Information (ESI) ForMariana FeijoNo ratings yet
- Group Meeting Problems 2023/04/08: O Me Me Me OH Me Me Me O HODocument7 pagesGroup Meeting Problems 2023/04/08: O Me Me Me OH Me Me Me O HOroccenxuNo ratings yet
- RearrangementsDocument45 pagesRearrangementssaheedvkNo ratings yet
- The Ethylene Acetal A Was Also Prepared by An Alternative ApproachDocument45 pagesThe Ethylene Acetal A Was Also Prepared by An Alternative Approachbann tvNo ratings yet
- A Second Route To The Key Tricyclic Intermediate A For The Synthesis of Gibberellic Acid Was Also Developed (Ref. 8)Document45 pagesA Second Route To The Key Tricyclic Intermediate A For The Synthesis of Gibberellic Acid Was Also Developed (Ref. 8)bann tvNo ratings yet
- Provide Reasonable Arrow-Push Mechanisms For Following RecationsDocument39 pagesProvide Reasonable Arrow-Push Mechanisms For Following RecationsCông Bằng NguyễnNo ratings yet
- Coumarin TOLTERODINEDocument9 pagesCoumarin TOLTERODINEJignesh TrivediNo ratings yet
- Assignment - 1 - FuransDocument2 pagesAssignment - 1 - FuransJitendra Kumawat 17115No ratings yet
- Exercise 14 - Carbonyl Chemistry: Claisen, Aldol Type-And 1,4-AdditionsDocument2 pagesExercise 14 - Carbonyl Chemistry: Claisen, Aldol Type-And 1,4-AdditionsAllalannNo ratings yet
- Research Article: Electrochemical Studies For Cation Recognition With Diazo-Coupled Calix (4) ArenesDocument8 pagesResearch Article: Electrochemical Studies For Cation Recognition With Diazo-Coupled Calix (4) ArenesNur AsyifaNo ratings yet
- Monte-Carlo Methods For Simulating The Catalytic Oxidative Dehydrogenation of Propane Over Vmgo CatalystDocument10 pagesMonte-Carlo Methods For Simulating The Catalytic Oxidative Dehydrogenation of Propane Over Vmgo CatalystMayteNo ratings yet
- XXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973From EverandXXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973No ratings yet
- Flojetpump HandleidingDocument4 pagesFlojetpump HandleidingnitroboozterNo ratings yet
- TSS-TI-021-02 Chalking of Epoxy Surfaces PDFDocument2 pagesTSS-TI-021-02 Chalking of Epoxy Surfaces PDFYeoh chun yenNo ratings yet
- Competition & Luxury Vehicle Club of Darlington SuitDocument31 pagesCompetition & Luxury Vehicle Club of Darlington SuitBenjamin DuerNo ratings yet
- DroperidolDocument1 pageDroperidolIvanne HisolerNo ratings yet
- RP12E Toc PDFDocument10 pagesRP12E Toc PDF황산악No ratings yet
- Chapter 13: SolutionsDocument18 pagesChapter 13: SolutionsBSNo ratings yet
- KInatics Theory and Atom and Nuclie MMDocument1 pageKInatics Theory and Atom and Nuclie MMSanjay GuptaNo ratings yet
- Por Si Te Puede ServirDocument7 pagesPor Si Te Puede ServirJordi ClaudioNo ratings yet
- Vacuum Testing of Fixed Roof Welded Storage Tanks As Per API 650,620Document7 pagesVacuum Testing of Fixed Roof Welded Storage Tanks As Per API 650,620Rakesh RanjanNo ratings yet
- The Mole Volume Relationships of GasesDocument15 pagesThe Mole Volume Relationships of GasesMaku MichaelNo ratings yet
- Biodegradation of Keratin Waste Theory and Practical AspectsDocument13 pagesBiodegradation of Keratin Waste Theory and Practical AspectsRodrigo Lara100% (1)
- Division9 FinishesDocument159 pagesDivision9 FinishesJosh HabanNo ratings yet
- Report BeetrootDocument11 pagesReport BeetrootSya Subi100% (3)
- OAP ExamDocument13 pagesOAP ExamCarmelita F. Cadapan100% (1)
- Rociadores - FT - GFS-100B - GL SeriesDocument2 pagesRociadores - FT - GFS-100B - GL SeriesJimmy FernándezNo ratings yet
- Scie 7 Q1 Module 2 WEEK 3Document11 pagesScie 7 Q1 Module 2 WEEK 3Dionne Sebastian DoromalNo ratings yet
- Lied Mann 2017Document8 pagesLied Mann 2017Chandra SekarNo ratings yet
- United States PatentDocument7 pagesUnited States PatentMichelle PatiñoNo ratings yet
- Mechanical Properties of Ultra-High-Performance Concrete Enhanced With Graphite Nanoplatelets and Carbon NanofibersDocument10 pagesMechanical Properties of Ultra-High-Performance Concrete Enhanced With Graphite Nanoplatelets and Carbon NanofibersRUSNA KPNo ratings yet
- Octostat 50Document1 pageOctostat 50chayanunNo ratings yet
- 1027 Application GuidelineDocument6 pages1027 Application GuidelineJORGEALEXERNo ratings yet
- MOLECONCEPTREDOXREACTIONCOMPLETEPACAKGEDocument52 pagesMOLECONCEPTREDOXREACTIONCOMPLETEPACAKGENikhil PalNo ratings yet
- Sizing Up The Valve Guide: by Dave MonyhanDocument4 pagesSizing Up The Valve Guide: by Dave MonyhanRidwanUsmanNo ratings yet
- Steel SAE O-Rings AdaptersDocument3 pagesSteel SAE O-Rings Adaptersgeav25653855No ratings yet
- Group #4: September 25, 2019Document5 pagesGroup #4: September 25, 2019MIKAYLA ELAINE P. DELA CRUZNo ratings yet
- 몽중1 P&ID 131227-제본파일 (링크 마크업Document272 pages몽중1 P&ID 131227-제본파일 (링크 마크업Lê Thành Chung100% (3)
- INOVYN™ PVC - Emulsion PVCDocument9 pagesINOVYN™ PVC - Emulsion PVCM Waheed AtharNo ratings yet
- Chapter-1 Ishida2011Document79 pagesChapter-1 Ishida2011sasidharkanthetiNo ratings yet
- Protein Sample Preparation & QuantificationDocument19 pagesProtein Sample Preparation & QuantificationKurdianto MSiNo ratings yet