Professional Documents
Culture Documents
Precision and Accuracy Activity
Uploaded by
Lloyd GarayCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Precision and Accuracy Activity
Uploaded by
Lloyd GarayCopyright:
Available Formats
Activity: Precision and accuracy
ACTIVITY: Precision and accuracy
Activity idea
In this activity, students analyse sets of data and decide whether the measurements are
precise and/or accurate.
By the end of this activity, students should be able to:
• define the terms ‘precision’ and ‘accuracy’
• use the terms ‘precision’ and ‘accuracy’ in a scientific setting
• analyse a measurement data set and judge its precision and accuracy.
Introduction/background
In this activity, student analyse sets of data and make statements about precision and
accuracy.
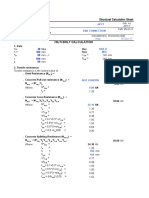
When taking scientific measurements, the goals are to measure accurately and with precision.
• Accuracy indicates the closeness of the measurements to the true or accepted value.
• Precision is the closeness of the results to others obtained in exactly the same way.
What you need
• Copies of the student worksheet: Analysing data
What to do
1. Hand out copies of the student worksheet: Analysing data. Read through the first page
together and discuss.
2. Have students work through the data analysis activities and discuss the results.
© Copyright. Science Learning Hub, The University of Waikato. 1
http://sciencelearn.org.nz
Activity: Precision and accuracy
Analysing data
When taking scientific measurements, the goals are to measure accurately and with precision.
• Accuracy indicates the closeness of the measurements to the true or accepted value.
• Precision is the closeness of the results to others obtained in exactly the same way.
In this image, the bull’s-eye represents the accepted true value. Each cross represents a
repeated measurement of the same quantity.
In certain situations in the laboratory, you may be measuring a quantity that has an accepted
value. The difference between the measured result and the accepted value is the error in the
result.
• error = measured value - accepted value
• % error = (error in measurement/accepted value) x 100
For example, the accepted value for the mass of a new golf ball is 45.93 g. A student weighs a
golf ball and finds it to be 46.45 g. The % error is:
% error = (error in measurement/accepted value) x 100
= [(46.45 – 45.93)/45.93] x 100
= [0.52/45.93] x 100
= 1.13%
Rather than measuring once, it is common practice to take a number of separate
measurements. This eliminates any ‘outlier’ results (results that do not fit the expected
outcome) and allows for an average value to be taken.
For example, a student weighs a golf ball on five separate occasions, and the results are:
45.89 g, 45.91 g, 46.06 g, 36.98 g and 45.94 g. On looking carefully at the results, it is clear
that the result ‘36.98 g’ does not fit in with the other results. It is an ‘outlier’ and is discarded.
The average mass is calculated as:
(45.89 + 45.91 + 46.06 + 45.94)/4 = 45.95 g
© Copyright. Science Learning Hub, The University of Waikato. 2
http://sciencelearn.org.nz
Activity: Precision and accuracy
Coin diameter
A 1-peso coin has an ‘accepted’ diameter of 24 mm.
Measure the diameter of the coin. Student A uses a simple plastic ruler. Student B uses a
precision measuring tool called a caliper. Have four trials in measuring the coin.
Student A – plastic ruler Student B – caliper
1. Calculate the average value for each set of measurements
Student A – plastic ruler Student B – caliper
2. Calculate the % error for each set of measurements.
Student A – plastic ruler Student B – caliper
3. Compare the average value for each set with the accepted value:
• Which student’s data is more accurate?
• Which student’s data is more precise?
4. Compare the percentage error for each set:
• Which student’s data is more accurate?
• Which student’s data is more precise?
5. Explain any odd findings:
© Copyright. Science Learning Hub, The University of Waikato. 3
http://sciencelearn.org.nz
Activity: Precision and accuracy
Aluminum, iron, brass, and copper
4 materials are given to each group namely: Iron sphere, Iron brass, Cylinder Copper, and
Aluminium cube. Each group is tasked to compute the volume of each material using the tools
provided by your facilitator. Each material must be measured four times and list the computed
volume as Trial 1, 2, 3, and 4.
Student A is told to use a simple plastic ruler and to make four independent measurements for
each dimension. Student B is told to use a precision measuring tool called a caliper.
Student A – plastic ruler Student B – caliper
1. Calculate the average value for each set of density values, making sure that any ‘outliers’
are not included.
Student A – plastic ruler Student B – caliper
2. Calculate the % error for each set of values.
Student A – plastic ruler Student B – caliper
3. Compare the average value for each set with the accepted value:
• Which student’s data is more accurate?
• Which student’s data is more precise?
4. Compare the percentage error for each set:
• Which student’s data is more accurate?
• Which student’s data is more precise?
5. Explain any odd findings:
© Copyright. Science Learning Hub, The University of Waikato. 4
http://sciencelearn.org.nz
You might also like
- 601 4 Research Reliability & ValidityDocument13 pages601 4 Research Reliability & ValidityDe ZerNo ratings yet
- GenAI Tools For PMs 091523Document6 pagesGenAI Tools For PMs 091523neethinathan.citrusNo ratings yet
- Hilti HSA AnchorsDocument4 pagesHilti HSA AnchorsandreashendiNo ratings yet
- Reliability & ValidityDocument33 pagesReliability & ValidityKhalilahanum Zainal Abidin100% (1)
- Y9 Science Transition Test Teacher GuideDocument30 pagesY9 Science Transition Test Teacher Guidekarendbrooks0% (1)
- Science 8 - Q4Document15 pagesScience 8 - Q4Lance Delos SantosNo ratings yet
- Accelerated Learning 3 - Foster - Basil Speed Reading and Memory Training Super Skills - Read Fast - FasDocument208 pagesAccelerated Learning 3 - Foster - Basil Speed Reading and Memory Training Super Skills - Read Fast - Fasger rivNo ratings yet
- Bruce Copen - Agricultural Radionics (OCR)Document43 pagesBruce Copen - Agricultural Radionics (OCR)evandrojsilva75% (8)
- Cosmetics - Research Report For MBADocument88 pagesCosmetics - Research Report For MBAvarunibmt77% (74)
- Authentic Assessment and Assessment of Process and ProductDocument23 pagesAuthentic Assessment and Assessment of Process and Productlaren100% (1)
- PM2 ToolsDocument68 pagesPM2 ToolsThảo Nguyên TrầnNo ratings yet
- Worksheet-Accuracy and Precision-FinalDocument4 pagesWorksheet-Accuracy and Precision-FinalSabeeh Ul HassanNo ratings yet
- Validity and Reliability of Research InstrumentDocument47 pagesValidity and Reliability of Research Instrumentjeffersonsubiate100% (4)
- Measurements Assessment and Evaluation in Outcome Based EducationDocument14 pagesMeasurements Assessment and Evaluation in Outcome Based EducationJoshua Poblete Directo67% (6)
- Monarch TT 30Document4 pagesMonarch TT 30Migue ToasaNo ratings yet
- Measurement, Assessment and Evaluation in Outcomes-Based EducationDocument59 pagesMeasurement, Assessment and Evaluation in Outcomes-Based EducationJohn Marvin CanariaNo ratings yet
- Precision and AccuracyDocument6 pagesPrecision and AccuracyAnna Lyse MosesNo ratings yet
- ACTIVITY: Precision and AccuracyDocument6 pagesACTIVITY: Precision and AccuracyRommel PápicaNo ratings yet
- LAS 1 Accuracy Vs Precision Errors and Uncertainties ContinuationDocument27 pagesLAS 1 Accuracy Vs Precision Errors and Uncertainties ContinuationFlor de Alda100% (1)
- Module 2Document10 pagesModule 2Kenth Godfrei DoctoleroNo ratings yet
- Gen Phy. Module 2Document14 pagesGen Phy. Module 2Ronin100% (1)
- Topic 8F Validity Reliability and Sources of ErrorDocument24 pagesTopic 8F Validity Reliability and Sources of ErrorCarleen Grace DeleonNo ratings yet
- Accuracy vs. Precision: Their Own Copy) and Have It Approved by The Teacher BEFORE You Write The Procedure in Your LabDocument3 pagesAccuracy vs. Precision: Their Own Copy) and Have It Approved by The Teacher BEFORE You Write The Procedure in Your LabantoinnevongenesisNo ratings yet
- Stress and Coping Scale Validity ReliabilityDocument2 pagesStress and Coping Scale Validity ReliabilityJohn ArthurNo ratings yet
- Properties of Assessment Method: ValidityDocument30 pagesProperties of Assessment Method: ValidityDANIEL LANCE RESPONTE NEVADONo ratings yet
- M 1 Testing Eval s1Document19 pagesM 1 Testing Eval s1Sou SouNo ratings yet
- Multiple Choice Test Item AnalysisDocument26 pagesMultiple Choice Test Item Analysiseen widyaselawekiaNo ratings yet
- Research 9 Module 5 Data Collection and MethodsDocument13 pagesResearch 9 Module 5 Data Collection and MethodsCody AngeloNo ratings yet
- Week 9 Lesson PR2Document25 pagesWeek 9 Lesson PR2Charles AnicieteNo ratings yet
- Unit-I: Meaning of Test, Measurement and EvaluationDocument14 pagesUnit-I: Meaning of Test, Measurement and Evaluationpepz funnelNo ratings yet
- Orientation Programme For Form 1 StudentsDocument24 pagesOrientation Programme For Form 1 StudentsVictor ManivelNo ratings yet
- C C: A P T: The Center On Learning, Assessment, and School StructureDocument6 pagesC C: A P T: The Center On Learning, Assessment, and School StructureDesilynQuibuyenVillanuevaNo ratings yet
- Analytical Problem Solving: by Gilbert PaceyDocument48 pagesAnalytical Problem Solving: by Gilbert PaceyjunomarsNo ratings yet
- New AssignmentDocument20 pagesNew AssignmentMyrnil TilbeNo ratings yet
- General Physics 1 (Week-1) MEASUREMENT (Accuracy and Precision)Document6 pagesGeneral Physics 1 (Week-1) MEASUREMENT (Accuracy and Precision)Mary graceNo ratings yet
- AIL Unit 3Document26 pagesAIL Unit 3julian.alipatNo ratings yet
- Whats Your AngleDocument42 pagesWhats Your AngleSyed Asim RazaNo ratings yet
- Fraenkel8 SMA Ch09Document9 pagesFraenkel8 SMA Ch09Pheru KhanNo ratings yet
- 1617syllabus BucholtzDocument5 pages1617syllabus Bucholtzapi-327307599No ratings yet
- Introductory Physics Students' Treatment of Measurement UncertaintyDocument21 pagesIntroductory Physics Students' Treatment of Measurement UncertaintyBhavesh TrivediNo ratings yet
- Unit 3 Spectroscopy Notes PDFDocument59 pagesUnit 3 Spectroscopy Notes PDF7nx58s9dyhNo ratings yet
- Chapter 1: Measurement: Accuracy, Precision, and ErrorDocument20 pagesChapter 1: Measurement: Accuracy, Precision, and Errorarya starkNo ratings yet
- Describe Adequately Quantitative Sampling Procedure and SampleDocument13 pagesDescribe Adequately Quantitative Sampling Procedure and SampleDarwisa NassalNo ratings yet
- Handouts - Accuracy VS PrecisionDocument9 pagesHandouts - Accuracy VS PrecisionfizznbitesNo ratings yet
- WBI06 01 Pef 20180124Document6 pagesWBI06 01 Pef 20180124Asem BarhoushNo ratings yet
- Review Lessons 1 2Document28 pagesReview Lessons 1 2api-235066492No ratings yet
- Ued 495-496 Ingerson Sarah Compa RationaleDocument4 pagesUed 495-496 Ingerson Sarah Compa Rationaleapi-278386840No ratings yet
- Force Motion EnergyDocument19 pagesForce Motion Energyapi-257978541No ratings yet
- Errors and Uncertainty in Experimental DataDocument12 pagesErrors and Uncertainty in Experimental DataKengkoy PascualNo ratings yet
- Teaching Inquiry: Teacher/Team: Cheyne Dowsett Year Level. 9 Date: 8/8/16Document10 pagesTeaching Inquiry: Teacher/Team: Cheyne Dowsett Year Level. 9 Date: 8/8/16api-332722181No ratings yet
- Lesson Plan Clas ViiDocument33 pagesLesson Plan Clas ViisharyNo ratings yet
- Test Bank For Statistical Concepts For The Behavioral Sciences 4 e 4th Edition 0205626246Document38 pagesTest Bank For Statistical Concepts For The Behavioral Sciences 4 e 4th Edition 0205626246inhoopnebuloseve9nqt100% (10)
- Measurement and Evaluation AnswersDocument29 pagesMeasurement and Evaluation Answersjimkuria90No ratings yet
- Chapter 3 PracreDocument11 pagesChapter 3 PracreRhandolfpaul CadeliñaNo ratings yet
- SP Iv-20Document4 pagesSP Iv-20sevynNo ratings yet
- Scientific ProcessDocument20 pagesScientific ProcessShyamili RajeevNo ratings yet
- General PhysicsDocument13 pagesGeneral PhysicsIca SafraNo ratings yet
- Enhanced Dodea Science Indicators 7th GradeDocument43 pagesEnhanced Dodea Science Indicators 7th Gradeapi-232424041No ratings yet
- Introduction To Reliability: What Is Reliability? Why Is It Important?Document14 pagesIntroduction To Reliability: What Is Reliability? Why Is It Important?sureshrnalNo ratings yet
- Compilation of Prelim Reports (Authentic Assessment and Rubrics)Document38 pagesCompilation of Prelim Reports (Authentic Assessment and Rubrics)RichardCastrenceParagasNo ratings yet
- Spss - PPT DR - MuthupandiDocument53 pagesSpss - PPT DR - Muthupandimskumar_meNo ratings yet
- Anero - Ped 8 Activity Sept. 11Document3 pagesAnero - Ped 8 Activity Sept. 11Jefher Ian AneroNo ratings yet
- 4 Principles of Language Testing Validity and Reliability of TestDocument48 pages4 Principles of Language Testing Validity and Reliability of Testmathpix2525No ratings yet
- PR2 1st QuarterDocument70 pagesPR2 1st Quarterjosel abendanioNo ratings yet
- Sir Quizana Res2 Chapter 3 Printed TemplateDocument12 pagesSir Quizana Res2 Chapter 3 Printed TemplateoliveNo ratings yet
- Precision and AccuracyDocument10 pagesPrecision and Accuracyeeiarias0503No ratings yet
- Measurement of Length - Screw Gauge (Physics) Question BankFrom EverandMeasurement of Length - Screw Gauge (Physics) Question BankNo ratings yet
- Safety Valve - 118CSS SpecificationDocument2 pagesSafety Valve - 118CSS SpecificationManuel Pimentel Del Campo100% (1)
- 4 - Classical Management 2Document64 pages4 - Classical Management 2Prof. Maseera PatelNo ratings yet
- Rr210106 Fluid MechanicsDocument8 pagesRr210106 Fluid MechanicsSrinivasa Rao GNo ratings yet
- CrashesDocument2 pagesCrashesInggritNo ratings yet
- Pressure Loss in Schedule 40 Steel PipesDocument14 pagesPressure Loss in Schedule 40 Steel PipesallovidNo ratings yet
- 3 Ps in Practice 8th Ed. Chapter 12 Page 456Document3 pages3 Ps in Practice 8th Ed. Chapter 12 Page 456Chitvan BhargavaNo ratings yet
- Create Database Permission DeniedDocument18 pagesCreate Database Permission DeniedlitpenguNo ratings yet
- Maastricht University Bachelor Thesis LawDocument8 pagesMaastricht University Bachelor Thesis Lawgjgt41ak100% (1)
- Executive SummaryDocument3 pagesExecutive SummaryPrashant Malhotra100% (1)
- DOL StartersDocument2 pagesDOL StartersAbhi TiwariNo ratings yet
- Afridi CV 2013Document13 pagesAfridi CV 2013seyed mohsen SalehiNo ratings yet
- Ict Project For Social ChangeDocument49 pagesIct Project For Social ChangeKyle RicardoNo ratings yet
- PhonemesDocument12 pagesPhonemesTsalits Muhammad NugrohoNo ratings yet
- Isocop Rev 07-11-2012 - ENGLDocument15 pagesIsocop Rev 07-11-2012 - ENGLAlexandru RucareanuNo ratings yet
- BSBLDR602 Assessment Task 2Document7 pagesBSBLDR602 Assessment Task 2GilsonVenâncioNo ratings yet
- Mayer+empl: Architectural Space InterventionDocument55 pagesMayer+empl: Architectural Space InterventionQuirin EmplNo ratings yet
- Major Assignment Brief (60%)Document14 pagesMajor Assignment Brief (60%)anees kausarNo ratings yet
- Program ATP EMTP in Overvoltage EducationDocument4 pagesProgram ATP EMTP in Overvoltage Educationrodrigoct88No ratings yet
- 40 Cau Part 5 Kem Loi Giai Chi TietDocument14 pages40 Cau Part 5 Kem Loi Giai Chi TietTrần DũngNo ratings yet
- Cisco Access Point Power RequirementsDocument5 pagesCisco Access Point Power RequirementsunivocalNo ratings yet
- Playlist ObserverDocument6 pagesPlaylist Observerricardogarcia1724gjNo ratings yet