Professional Documents
Culture Documents
Us3857926 NS 2
Uploaded by
dwikinovendra230 ratings0% found this document useful (0 votes)
4 views8 pagesOriginal Title
US3857926-NS-2
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
4 views8 pagesUs3857926 NS 2
Uploaded by
dwikinovendra23Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 8
United States Patent (19) (11) 3,857,926
Beutner et al. (45) Dec. 31, 1974.
(54) PRODUCTION OF NICKEL SULFATE Mellor, A Comprehensive Treatise on Inorganic and
75) Inventors: Heinz Paul Beutner, Englewood, Theoretical Chemistry, Vol. 5, 1924, p. 956.
Colo.; Charles Edward O'Neill, Port William E. Trout, Jr., Journal of Chemical Education,
Credit, Ontario, Canada; George December 1937, pp. 575-581.
Feick, Needham, Mass.
73) Assignee: The International Nickel Company, Primary Examiner-Oscar R. Vertiz
Assistant Examiner-Hoke S. Miller
Inc., New York, N.Y.
22 Filed: Mar. 26, 1973 (57) ABSTRACT
21 Appl. No.: 345,180 A process for producing nickel sulfate from nickel
carbonyl which comprises reacting nickel tetracarbo
(52) U.S. Cl.............................. ... 42.3/544, 423/393 nyl with nitric acid in the gaseous phase, absorbing the
51) int. Cl........................ C01g 53/10, CO lb 21140 solid product of the reaction in an aqueous solution of
58 Field of Search..................... 4231544, 417, 393 sulfuric acid and recovering nickel sulfate from the
aqueous solution of sulfuric acid. The process also
(56) References Cited contemplates an essentially closed system wherein ni
UNITED STATES PATENTS tric acid, nitrogen oxides and carbon monoxide are re
cycled. The carbon monoxide is reused to form nickel
3,256,060 6/1966 Glohus...... . . . . . . . . . . . . . . . . . . . . . . . . . . 423.1417 carbonyl. The nitrogen oxides are reoxidized to nitric
OTHER PUBLICATIONS acid and the nitric acid is reused to react with addi
Jacobson, Encyclopedia of Chemical Reactions, Vol. tional nickel carbonyl.
V, 1953, p. 16 and p. 35. 9 Claims, 2 Drawing Figures
PATENTED DEC3974 3,857,926
SHEET 2 OF 2
3,857,926
2
PRODUCTION OF NICKEL SULFATE contacting column consisting of a packed tower, which
is irrigated with an aqueous liquor containing in solu
The present invention is concerned with the manu tion sulfuric acid and advantageously maintained at a
facture of nickel sulfate and, more particularly, with temperature of about 70° or 80°C. up to about the boil
the manufacture of nickel sulfate from nickel carbonyl. ing point under the ambient pressure in the system of
It is well known that in processes of extracting nickel the aqueous liquor. Sulfuric acid enters into the reac
from its ores, one commercially important process in tion when the solid product of the gas phase reaction
volves the selective carbonylation of nickel-containing is absorbed by the irrigating liquor. After the reaction
material to form nickel tetracarbonyl (referred to here is complete, nickel sulfate is crystallized out of the li
inafter as "nickel carbonyl'). It would be highly advan O quor usually in the form of the hexahydrate.
tageous, and is genrally the object of the present inven While it is possible to carry out the process of the
tion, to convert nickel carbonyl directly into nickel present invention without providing any means for re
chemicals such as nickel sulfate. The formation of covery of materials used in the process, it is highly ad
nickel sulfate directly from nickel carbonyl is highly ad vantageous to provide means to recover and purify car
vantageous in that nickel carbonyl can easily be pre 15 bon monoxide, to recover nitric acid, oxidize nitrogen
pared as a very pure material. Consequently, it is ex oxides in the presence of water so as to reform nitric
pected that nickel sulfate prepared from nickel car acid and to minimize losses of any kind.
bonyl will have a very high degree of purity with re A schematic outline of such a system is depicted in
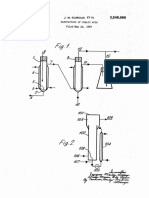
spect to other metallics. Obtaining such a high degree FIG. of the drawing.
of purity in nickel sulfate made by other methods some 20 Referring now thereto, FIG. 1 depicts a system hav
times involves considerable difficulty. ing four functional sections, reaction zone 11, nitric
It has now been discovered that by means of a specia acid recover zone 12, gas purifying zone 13, and prod
process nickel sulfate can be prepared nickel carbonyl uct recovery zone 14. In reaction zone 11, nickel car
efficiently and in excellent yield. bonyl gas reacts with gaseous nitric acid and is ab
It is an object of the present invention to provide a 25 sorbed in a circulating liquor containing sulfuric acid.
novel process for producing nickel sulfate sulfate from The circulating liquor is maintained advantageously at
nickel carbonyl. about 100° C. Gas exiting from reaction zone 11 and
It is another object of the present invention to pro entering nitric acid recovery zone 12 contains nitrogen
vide a novel process of providing nickel sulfate from 30 oxides and carbon monoxide. This gas is mixed with ox
nickel carbonyl wherein carbon monoxide, nitric acid, ygen and contacted by process makeup water in nitric
and nitrogen oxides employed in or produced in the acid recovery zone 12 to produce a dilute nitric acid
process are recovered and reused. solution which is returned to the circulating liquor sup
Other objects and advantages will become apparent ply. Gas purification zone 13 comprises a gas holdup
from the following description taken in conjunction zone which permits the oxidation of a substantial pro
35 portion of the remaining nitric oxide to nitrogen diox
with the drawing in which:
FIG. 1 shows a schematic outline of the novel process ide. The thus produced nitrogen dioxide is then scrub
of the present invention; and bed by sulfuric acid to dry the gas and to absorb a sub
FIG. 2 is a flow diagram of an advantageous aspect stantial portion of the nitrogen oxides present to pro
of the process of the present invention. 40
duce nitrosyl sulfuric acid. The gas exiting from the sul
Generally speaking, the present invention contem furic acid scrubbing means, contains small amounts of
plates a process of converting nickel carbonyl which nitrogen oxides and oxygen with the balance being es
comprises reacting the nickel carbonyl with nitric acid sentially carbon monoxide. The gas can be further puri
in the gaseous phase, absorbing the solid product of fied in conventional ways to remove the nitrogen ox
said reaction in an aqueous solution of sulfuric acid and 45 ides and the oxygen so as to produce essentially pure
recovering nickel sulfate from the aqueous solution of carbon monoxide. Means for removing nitrogen oxides
sulfuric acid. include a secondary scrubbing by sulfuric acid, a cata
The process of the present invention is represented lytic oxidation of nitric oxide on active charcoal or the
for purposes of material balance by the equation like. Oxygen can be removed by absorption on copper
Ni (CO) + 2% HNO + HSO -> Ni SO + 1 % HO lyst. 50 or conversion to carbon dioxide over a palladium cata
-- 4CO -- % NO Product recovery zone 14 comprises means for
cooling a part of the circulating liquor to crystallization
Assuming that nitrogen oxides are reoxidized and re temperature, means for crystallizing nickel sulfate
used the following material balance equation is applica means for recovering the crystals from the mother li
ble. 55 quor and means for returning the mother liquor to re
action zone 11.
Speaking more particularly with respect to the reac
Overall then, assuming that the nickel sulfate is recov tion zone 11, as maintained in a continuously oprating
ered as the hexahydrate, the material balance, assum system, it is necessary to provide a suitable circulating
ing no losses, is represented by the equation Ni (CO) 60 liquor composition which has certain criteria. The cir
4 O + HSO + 5 HO -> NiSO-6 HO + 4CO. culating liquor must contain sulfuric acid. It must give
Those skilled in the art will appreciate that the forego at the reaction temperature, e.g., 100°C., a sufficiently
ing equations set forth the material balance only and do high vapor pressure of nitric acid to provide nitric acid
not necessarily represent the mechanism of the process in the vapor phase in amounts adequate to react com
of the present invention. Contrary to the impression 65 pletely with in-coming nickel carbonyl. It must be capa
one might get from the first of the foregoing equations, ble of dissolving nickel sulfate; it must contain water
reaction occurs between nickel carbonyl and nitric acid and it must be capable of releasing crystals of nickel
mainly in the gas phase, advantageously in a gas liquid sulfate hexahydrate upon cooling, for example, down
3,857,926
3 4.
to 25 C. or below. A circulating liquor composition saddles or the like in the reaction zone to provide large
which has been found to be effective for the foregoing surface areas for liquid-gas interaction and thus the
purposes contains, in percent by weight, about 30% sul maintenance of a relatively high steady state concen
furic acid, about 5% nitric acid, about 10.6% nickel sul tration of nitric acid in the reaction zone atmosphere.
fate, calculated as the anhydrous salt, with the balance The oxygen consumed in the overall reaction and used
being water. Those skilled in the art will appreciate that specifically to reoxidize oxides of nitrogen produced by
at the initiation of the process, the circulating liquor reaction of nickel carbonyl and nitric acid can be intro
will not necessarily contain any significant amount of duced along with nickel carbonyl and nitric acid into
nickel. As the process proceeds, nickel sulfate will ac the gas space of reaction zone 11 or, alternatively or in
cumulate in the circulating liquor and when it exceeds O addition, can be introduced into the gas stream exiting
approximately 10%, measured as the anhydrous salt, from reaction zone 1 1. Unlike prior art endeavors
crystallization of the hexahydrate can occur attemper which in the past have produced explosions with mix
atures of about 5 C. At about 10.6% anhydrous nickel tures of nickel carbonyl and oxygen, the reactions in
sulfate, crystallization of the hexahydrate can occur at volved in the process of the present invention proceed
temperatures as high as 25 C. When the nickel sulfate 5 rapidly but smoothly at about 100° C. even in the pres
accumulates to the amount of about 13.8%, measured ence of oxygen in amounts as contemplated in the over
as the anhydrous salt, crystallization will occur at 92 all material balance equation set forth hereinbefore. It
C. Accordingly, in order to effectively operate the pro is believed that oxygen takes little or no part in the re
cess of the present invention on a continuous basis, it actions proceeding in reaction Zone 11 because appar
is advantageous to maintain the concentration of anhy 20 ently equivalent reactions take place both in the pres
drous nickel sulfate in the circulating liquor at abuot ence and absence of oxygen. However, to the extent
10% to about 13% by weight. This permits continuous oxygen may contribute to the reactions occurring in re
crystallization of part of the circulating liquor in a crys action zone. 11, such contribution is contemplated to be
tallizer at temperatures of about 25° C. without the within the purview and ambit of the claims of the pres
danger of crystals forming in the system at locations 25 ent application.
where the temperature is higher. For practical control The process of the present invention has been con
purposes, one should advantageously maintain the con ceived and reduced to practice as a process operating
centration of nickel sulfate, measured as the anhydrous under essentially normal atmospheric pressure. Nickel
salt, in the liquor at about 10.5% to 11.5% by weight. carbonyl has been introduced into reaction zone 11 in
While it is possible to use larger or smaller amounts 30 the form of vapor at about 0.5 atmosphere partial pres
of sulfuric acid and nitric acid in the circulating liquor, sure in carbon monoxide carrier gas, the two gases hav
it is highly advantageous to employ about 30% by ing a total pressure only sufficiently slightly above at
weight sulfuric acid and about 5% by weight of nitric mospheric pressure so as to insure entry into reactin
acid. The selection of these concentrations represents 35 zone 11. Obviously, pressures both higher and lower
a compromise between the desirability of a high acid than atmospheric can be used if desired. Pressures
concentration to increase the reactivity of the nitric lower than atmospheric may result in inefficient use of
acid with nickel carbonyl and the need for resonably space in the total system. Speaking solely of reaction
high solubility of nickel sulfate in the liquor attempera zone 11, it is to be noted that because the volume of ex
tures around 100° C. and a lesser solubility at lower iting gases is substantially greater than the volume of
temperature to allow product recovery by crystalliza 40 gases entering into the reactions in reactor zone 11, lit
tion. It is to be observed, however, that the process of tle advantage is to be gained from superatmospheric
the present invention is operable employing amounts of pressure operation. On the other hand, as a counterbal
sulfuric acid of about 20% to about 60% by weight and ancing feature the oxidation of nitrogen oxides in the
amounts of nitric acid of about 1% to about 40% by 45
presence of water to form nitric acid is clearly en
weight in the circulating liquor. Those skilled in the art hanced by high pressure. Accordingly, although com
will appreciate that the remainder of the circulating li plications may be involved, it is within the contempla
quor other than dissolved nickel sulfate is essentially tion of the present invention to provide a process
Water. wherein the reactions taking place in reaction zone 11
When employing the advantageous circulating liquor are conducted at or around one atmosphere of a pres
in accordance with the concepts of the present inven 50 sure and the reactions involved in oxidation of nitrogen
tion as discussed hereinbefore, which provides a partial oxides in nitric acid recovery zone 12 are conducted at
pressure of about one-half atmosphere of water at 100 higher pressures, for example, up to about 10 atmo
C. along with nitric acid vapor, a residence time of spheres or higher. Gas purification occurring in gas pu
about 10 seconds of nickel carbonyl in the reaction rifying zone 13 can also be carried out at superatmos
zone is all that is necessary to achieve reaction. Be 55 pheric pressure if desired.
cause it is important for reasons of both safety and eco In addition to means 15, 16, 17 and 18 for introduc
nomics that all nickel carbonyl entering reaction Zone ing reacting amounts of nickel carbonyl, oxygen sulfu
11 react with nitric acid it is necessary to maintain the ric acid and water, respectively, into the system de
rate of circulation of irrigating liquor so that a stoichio 60 picted in FIG. 1 of the drawing, means 19 is provided
metrical excess of nitric acid is present in the gas phase. for introducing makeup amounts of nitric acid into the
Ordinarily this will be satisfied when operating at 100 system and means 20 is provided to exhaust carbon
C. by introducing at least about 10 and up to about 200 monoxide from the system. While nitric acid functions
or more mole weights of nitric acid in the irrigating li as a reformable oxidizing agent and thus is catalytic in
quor into the reaction zone for each mole weight of 65 nature in the process of the present invention, inevita
nickel carbonyl introduced into the reaction zone. The ble losses of nitric acid occur chiefly because it is unec
foregoing teaching concerning the required amount of onomic to reoxidize all nitrogen oxides produced by re
circulating liquor presumes, of course, the use of rings, action of nitric acid with nickel carbonyl. Small
S
3,857,926
6
amounts of nitrogen oxides are removed in gas purifica by weight sulfuric-acid-in-water irrigating liquid men
tion zone 13 without reoxidation and recovery. Ac tioned in the preceding paragraph in place of an equal
cordingly, makeup amounts nitric acid equivalent to . percent by weight of water and changing nothing else
the losses of nitrogen oxides should be introduced into resulted in a smooth reaction in the gas-liquid contact
the system depicted in FIG. 1. Also depicted in FIG. 1, unit and build up of nickel sulfate in the irrigating liq
oxygen admitted to the system by means 16 can be ad uid.
mitted to reaction zone or to nitric acid recovery
zone 2 or at both locations. EXAMPLE V
In order to give those skilled in the art a better under An apparatus was set up comprising a heated reser
standing and appreciation of the invention, the follow 10 voir maintained at about 100°C., a gas-liquid raschig
ing Examples are given: ring-packed gas-liquid contacting column surmounting
EXAMPLE I and connected to the reservoir, a pump and line
adapted to draw liquor from the reservoir and inject it
A reaction vessel having a vertically standing gas at the top of the gas-liquid contacting column, a reflex
liquid contact unit containing Rachig rings and having 15 condensor connected to the top of the gas-liquid con
a volume of 500 units was set up for counter-current tacting column, a gas purifying train connected to the
liquid-gas flow at a temperature of 100° C. and atmo outlet of the reflex condensor and venting to the atmo
spheric pressure. An irrigating liquid initially contain sphere, oxygen feed means to the bottom of the gas
ing 45% by weight sulfuric acid, 5% by weight of nitric liquid contacting column and on to the stream of gas
acid with the balance water and heated to 100° C. was 20 exiting at the top of the gas-liquid contacting column
caused to flow rapidly downward through the contact a carbon monoxide feed means to the bottom of the
unit. Simultaneously, a gas mixture containing 25 parts gas-liquid contacting column and a nickel carbonyl va
by volume of carbon monoxide, 25 parts by volume of porizing and feeding means as a controllable shunt on
nickel tetracarbonyl and 50 parts by volume of oxygen the carbon monoxide feed means. The apparatus also
was introduced at a rate of 66 parts by volume per min 25 included means for sampling and monitoring the irri
ute into the bottom of said gas-liquid contact units and gating liquor and recovering crystalline product there
caused to flow toward a top exit. Essentially all of the from. A number of runs were made using the aforedes
nickel carbonyl reacted in the gas-liquid contact unit so cribed apparatus with a gas feed calculated to give a 10
as to produce an exit gas enriched in carbon monoxide second nickel carbonyl residence time in the gas-liquid
and containing nitrogen oxides. Nickel sulfate hexahy 30 contacting column. Simultaneously, during the runs,
drate was crystallizable from the irrigating liquid. the gas-liquid contacting column was irrigated with a
EXAMPLE II liquor containing 37.2% by weight sulfuric acid, 9.5%
by weight HNO and about 10% by weight of nickel sul
In a reaction vessel similar to that used in Example 35
fate (calculated as the anhydrous salt) with the balance
I but having a gas-liquid contact unit of 400 unit vol being water at a rate such that a volume of liquor ap
ume maintained at about 100° C., an irrigating liquid proximately 1.5 times the volume of the gas-liquid con
containing 25% by weight of sulfuric acid, 25% by tacting column was passed therethrough every minute.
weight of nitric acid, 8.6% by weight of nickel sulfate Using a 50 volume percent feed of nickel carbonyl in
(based on anhydrous salt) with the balance water was 40 carbon monoxide and no oxygen addition, it was found
rapidly flowed counter-current to a gas containing that about 6.1 moles of exit gas are produced for each
equal parts by volume of nickel carbonyl and carbon mole of nickel carbonyl reacted, the exit gases analyz
monoxide flowing at a rate of 40 volume units per min ing at about 5.4 moles of carbon monoxide to about
ute. Reaction over a period of 120 minutes at atmo 0.66 mole of nitric oxide. No nickel carbonyl was de
spheric pressure resulted in 100% reaction in the gas 45
tected in the exit gases indicating complete reaction in
liquid contact unit and recoverable nickel sulfate hexa a 10 second residence time in the gas-liquid contacting
hydrate in the irrigating liquid. column. In runs where oxygen was added either at the
EXAMPLE III base of the gas-liquid contacting column or at the exit
thereof, very little nitric oxide was found in the exit
Similar results were obtained to those set forth in Ex gases, the bulk thereof being oxidized to nitrogen diox
ample II employing the same apparatus, the same gas 50 ide or other higher oxides. This shows that presence of
mixture, the same gas and liquid flow rates and the oxygen in the nickel carbonyl reaction zone is unneces
same temperature; the only difference being that the sary for oxidation of nitric oxide. In the runs, nickel
irrigating liquid contained (in percent by weight) about containing product was recovered both from the liquor
45% sulfuric acid, about 5% nitric acid, about 8.6% 55 and mist carried over from the gas-liquid reacting col
nickel sulfate, (based on anhydrous salt) with the bal umn. The product crystallized from the liquor was
ance being essentially water. nickel sulfate hexahydrate. The product carried over as
In contrast to the foregoing Examples, a test run in mist from the top of the gas-liquid contacting column
the same type of apparatus at the same temperature was free of sulfate indicating that sulfuric acid does not
and pressure using 50% by weight sulfuric acid in water 60 significantly enter into the reactions inherent in the
as the irrigating liquid and a gas mxiture of 40 volume process of the present invention until the product of the
percent carbon monoxide, 40 volume percent nickel reaction of nitric acid and nickel carbonyl is absorbed
carbonyl and 20 volume percent oxygen resulted in an in the circulating liquor.
explosive reaction either in the exhaust gas or in the re
actO. 65 EXAMPLE VI
EXAMPLE IV A commerical size installation suitable for operating
the process of the present invention on a commercial
Addition of 4.2% by weight of nitric acid to the 50% scale is depicted in schematic outline in FIG. 2 of the
3,857,926
7 8
drawing. Referring now thereto, columnar reactor 21 mixture passes out of nitrogen oxides absorber 50 by
comprises from bottom to top well 22 containing, dur means of line 52 and is pumped by means of pump 53
ing operation, hot (100° C.) irrigating liquor having a to and into the top of columnar reactor 21. Tail gas ex
steady state composition of about 30% by weight sulfu iting from nitrogen oxides absorber 50 through line 54
ric acid, about 5% by weight nitric acid, about 10.6% is essentially dry and contains greater than 98% by vol
by weight nickel sulfate (based on anhydrous salt) with ume carbon monoxide Residual impurities can be re
the balance being water; means 15 for introducing moved by absorbing or catalytic means prior to reuse
nickel carbonyl plus carrier gas, e.g., carbon monoxide, of the carbon monoxide in the manufacture of nickel
into columnar reactor 21; liquid gas contacting volume carbonyl or for other purposes.
23 packed with raschig rings, saddles or the like; spray O Those skilled in the art will appreciate that the appa
means 24; condensor 25; liquid-gas contact volume 26 ratus depicted in FIG. 2 of the drawing and referred to
and mist eliminator 27. Irrigating liquid in well 22 is in Example VI can be modified somewhat and still
caused to flow through line 28 by circulating pump 29 achieve the advantages of the present invention. Thus,
through heat exchanger 30 (fed with steam through the apparatus used in carrying out the process of the
line 31 and exhausted by condensate line 32) into co 15 present invention comprises essentially means for re
lumnar reactor 21 at a point above packed volume 23 acting nickel carbonyl with nitric acid in the gaseous
and below condensor 25. Spray means 24 allows effi phase, means for absorbing the nickel-containing reac
cient wetting of the packing in volume 23 by irrigating tion product in a sulfuric acid-containing medium and
liquid flowing through line 28. Condensor 25 is sup means for recovering nickel sulfate from said medium.
plied internally with cooling fluid by means of line 33, 20 In addition to this core apparatus, means can be pro
circulating pump 34 and heat exchanger 35 supplied vided for oxidizing nitrogen oxide products of the
with cold water by means of lines 36 and 37. On a con nickel carbonyl-nitric acid reaction, for recycling the
tinuous basis part of the irrigating liquid flowing in line reoxidized nitrogen oxides as nitric acid in the reaction
28 is diverted through line 38 into crystallizer 39, the as well as for purifying carbon monoxide used as a car
irrigating liquid depleted in nickel sulfate being re 25 rier and produced in the reaction. All means capable of
turned to line 28 through line 40. Nickel sulfate hexa performing these functions are to be considered to be
hydrate is removed from crystallizer 39 through prod within the ambit and scope of the present invention.
uct take-off 41. For example, while the invention has been described in
During operation of columnar reactor 21, gases rich 30
terms of product recovery by crystallization of nickel
in carbon monoxide and containing nitrogen oxides sulfate hexahydrate, other means of recovery are con
(e.g., nitric oxide), water vapor and nitric acid vapor, templated. For example, with an irrigating liquor in
exit from columnar reactor 21 by means of line 42 and which nickel sulfate has low solubility it is possible to
are caused to enter nitric acid column 43 at the bottom recover product therefrom by continuous filtation
thereof. Oxygen in amounts adequate to react with ni 35
CalS.
trogen oxides present on the basis of the material bal As a further point in the description in Example VI,
ance equations set forth hereinbefore is also caused to materials of construction have not been specified be
enter nitric acid column 43 at the bottom thereof cause, as those skilled in the art will appreciate, a wide
through means 16. Nitric acid column 43 comprises an variety of acid resisting materials are readily available
enclosure containing a series of bubble cap plates. 44 40
for each usage. Among such materials are chemical
which are fed from the top by water entering through stoneware, porcelain, glass-lined steel equipment and
means 18 and line 44. Nitric acid diluted with water lines, steel coated with polytetrafluoroethylene, stain
exits from the bottom of nitric acid tower 43 through less steel and the like.
line 45 and is forced by pump 46 through line 45 to and Although the present invention has been described in
into the top of columnar reactor 21. Makeup amounts 45
conjunction with preferred embodiments, it is to be un
of nitric acid to compensate for losses of nitrogen ox derstood that modifications and variations may be re
ides are caused to enter the top of nitric acid column sorted to without departing from the spirit and scope of
43 through means 19 shown to be cut off by valve 46 the invention as those skilled in the art will readily un
to indicate that makeup amounts of nitric acid can be derstand. Such modifications and variations are consid
added intermittently. 50 ered to be within the purview and scope of the inven
Gases exiting from nitric acid tower 43 are rich in tion and appended claims.
carbon monoxide and contain small amounts of unre We claim:
acted oxygen, nitric oxide and water vapor. These gases 1. A process for producing nickel sulfate comprising
pass through line 47 into nitric oxide oxidation column reacting nickel carbonyl with nitric acid in the gas
48 containing free space adequate to provide a gas resi 55 phase, absorbing the nickel-containing product of said
dence time sufficient to allow significant additional oxi reaction in an aqueous solution of sulfuric acid and re
dation of nitric oxide. Gases exiting from nitric oxide covering nickel sulfate from said aqueous solution.
oxidation column consisting chiefly of carbon monox 2. The process as in claim 1 wherein the reaction be
ide, water vapor, oxygen, and small, essentially equi tween nickel carbonyl and nitric acid is carried out at
molar amounts of nitric oxide and nitrogen dioxide. 60 a temperature of at least about 70° C.
These gases pass through line 49 into the bottom of ni 3. The process as in claim 1 wherein the reaction be
trogen oxides absorber 50. Nitrogen oxides absorber 50 tween nickel carbonyl and nitric acid is carried out in
comprises a ring-or saddle-packed volume 51 over a gas phase in contact with hot aqueous solution con
which packing passes gases entering through line 49 taining nitric acid in addition to sulfuric acid.
and concentrated sulfuric acid entering through means 65 4. The process as in claim 3 wherein the aqueous so
17. The sulfuric acid absorbs essentially all the water lution is maintained at about about 100° C. and con
vapor in the gases and a large amount of the nitrogen tains, in percent by weight, about 20% to about 60% of
oxides to form nitrosyl sulfuric acid. The diluted acid sulfuric acid and about 1% to about 40% nitric acid.
3,857,926
9 10
5. The process as in claim 4 wherein the aqueous so by crystallization as nickel sulfate hexahydrate.
lution is containuously circulated through a liquid 8. The process as in claim 1 operated continuously
phase gas-phase contact zone at about 100° C. and con wherein makeup amounts of sulfuric acid are added in
tains as a steady state composition in percent by weight the form of concentrated acid brought into contact
about 30% sulfuric acid, about 5% nitric acid, about with gaseous products of the reaction of nickel car
10% to about 13% nickel sulfate (measured as the an bonyl and nitric acid to dry said gaseous products and
hydrous salt) with the balance being essentially water. absorb significant quantities of nitrogen oxides there
6. The process as in claim 1 wherein nitrogen oxides from.
resulting from the reaction of nitric acid and nickel car 9. The process as in claim 1 wherein the carbon mon
bonyl are reacted with oxygen in the presence of water 1O oxide fraction of the gaseous products of the reaction
to form nitric acid for reaction with additional nickel of nickel carbonyl and nitric acid is purified and caused
carbonyl. to react with nickel to provide additional nickel car
7. The process as in claim 1 wherein nickel sulfate is bonyl.
recovered from said aqueous solution of sulfuric acid ck k k k sk
15
25
30
35
40
45
50
55
60
65
You might also like
- Chemical Engineering Reviewer EditedDocument346 pagesChemical Engineering Reviewer EditedCatriona Black100% (2)
- Production of Sodium Chlorite PDFDocument13 pagesProduction of Sodium Chlorite PDFangelofgloryNo ratings yet
- Us3869257 NS 1Document6 pagesUs3869257 NS 1dwikinovendra23No ratings yet
- United States Patent: (10) Patent N0.: (45) Date of PatentDocument4 pagesUnited States Patent: (10) Patent N0.: (45) Date of PatentrezaroohollahiNo ratings yet
- United States Patent (191 (11) Patent Number: 5,270,023: May Et A1. (45) Date of Patent: Dec. 14, 1993Document8 pagesUnited States Patent (191 (11) Patent Number: 5,270,023: May Et A1. (45) Date of Patent: Dec. 14, 1993Yustinus Selis ToronNo ratings yet
- US4495107ADocument2 pagesUS4495107AWojciech RedutkoNo ratings yet
- US4668495Document5 pagesUS4668495Chemist Ahmed FoudaNo ratings yet
- 1936 - Vosburgh, Israel, Birch - The System Nickel Oxalate, Potassium Oxalate and Water at 30°Document2 pages1936 - Vosburgh, Israel, Birch - The System Nickel Oxalate, Potassium Oxalate and Water at 30°katiussdjNo ratings yet
- Coke Formation Onsilica-Alumina Cracking CatalystsDocument6 pagesCoke Formation Onsilica-Alumina Cracking Catalystsดั๊มพ์ วาสนาทิพย์No ratings yet
- US2343534 (Proses Sulit)Document4 pagesUS2343534 (Proses Sulit)aris_nurhidayatNo ratings yet
- By /r26 Ézi-44: Feb. 2, 1960 K. Falk Eta 2,923,728Document5 pagesBy /r26 Ézi-44: Feb. 2, 1960 K. Falk Eta 2,923,728masood kblNo ratings yet
- Lead chamber process: Industrial method for sulfuric acidDocument3 pagesLead chamber process: Industrial method for sulfuric acidMuhammad Bilal100% (2)
- @ Gradeff US3869517 1975 Process For Preparing Hydroxy Citronellal Via 1,1-DiacetatesDocument10 pages@ Gradeff US3869517 1975 Process For Preparing Hydroxy Citronellal Via 1,1-DiacetatesLouisNo ratings yet
- A New Method For Nitration of Phenolic CompoundsDocument6 pagesA New Method For Nitration of Phenolic CompoundsOmar valdesNo ratings yet
- Us 4378342Document9 pagesUs 4378342هیمن مNo ratings yet
- '" Is "S" 165,443 3/1965 U.S.S.R. 260/531: United States Patent 15 3,678,107Document5 pages'" Is "S" 165,443 3/1965 U.S.S.R. 260/531: United States Patent 15 3,678,107budispartanNo ratings yet
- US4119502Document5 pagesUS4119502sheenat100No ratings yet
- Continuous process for producing propylene oxide from propylene chlorohydrinDocument8 pagesContinuous process for producing propylene oxide from propylene chlorohydrinWidya Isti AriantiNo ratings yet
- Oct. 23, 1969 M. H. Maurer Et All 3,475,120: Filed March 28, 1967 2 Sheets-Sheet LDocument9 pagesOct. 23, 1969 M. H. Maurer Et All 3,475,120: Filed March 28, 1967 2 Sheets-Sheet LGraciaVelitarioNo ratings yet
- A Convenient Preparation of Volatile Acid ChloridesDocument4 pagesA Convenient Preparation of Volatile Acid ChloridesScott SwartzNo ratings yet
- Adkins 1949Document5 pagesAdkins 1949Falih RezkiNo ratings yet
- @ Gradeff US3917713 1975 Process For Preparing HydroxycitronellalDocument9 pages@ Gradeff US3917713 1975 Process For Preparing HydroxycitronellalLouisNo ratings yet
- Nitric Acid Manufacturing ProcessesDocument55 pagesNitric Acid Manufacturing ProcessesBhavesh Dilip ChanchlaniNo ratings yet
- Us 3549696Document4 pagesUs 3549696budispartanNo ratings yet
- Show Catalyst PDFDocument6 pagesShow Catalyst PDFabhi100% (1)
- Lecture 16 Nitric Acid PDFDocument11 pagesLecture 16 Nitric Acid PDFKuldeep Bhatt100% (1)
- Heterogeneous catalyst design for liquid-gas oxidationDocument4 pagesHeterogeneous catalyst design for liquid-gas oxidationChi Wing TsangNo ratings yet
- And 2,5-Dimethyltetrahydrofuran: J. PilgrimDocument3 pagesAnd 2,5-Dimethyltetrahydrofuran: J. PilgrimEdy MorarNo ratings yet
- Carbon DisulfideDocument12 pagesCarbon DisulfideMelissa Daniela Romero TrujilloNo ratings yet
- United States Patent (19) : Attorney-Harry M. Saragovitz, Edward J. Kelly, HerDocument7 pagesUnited States Patent (19) : Attorney-Harry M. Saragovitz, Edward J. Kelly, Herwilly dwinovNo ratings yet
- Carbon DisulfideDocument12 pagesCarbon DisulfideMelissa Daniela Romero TrujilloNo ratings yet
- United States Patent: 1,251,202 12/1917 Ellis.............................. 260/667Document6 pagesUnited States Patent: 1,251,202 12/1917 Ellis.............................. 260/667Septi WidyaNo ratings yet
- United States Patent (19) : 11 Patent Number: 45) Date of PatentDocument7 pagesUnited States Patent (19) : 11 Patent Number: 45) Date of PatentOrquídea McLoughlin SantanaNo ratings yet
- Ajoc201700267 PDFDocument11 pagesAjoc201700267 PDFluispedro1985No ratings yet
- Oxalic Acid Via Nitric Acid Oxidation of Hardwood Red OakDocument11 pagesOxalic Acid Via Nitric Acid Oxidation of Hardwood Red Oaknurlayli amanahNo ratings yet
- Process for Deodorizing Isopropyl Alcohol Using Silver-Treated Cation Exchange ResinDocument4 pagesProcess for Deodorizing Isopropyl Alcohol Using Silver-Treated Cation Exchange ResinMani ChemistNo ratings yet
- Us 6288289Document16 pagesUs 6288289ade sefliaNo ratings yet
- Us 3433584Document5 pagesUs 3433584Younes MahceneNo ratings yet
- Process For The Extraction andDocument11 pagesProcess For The Extraction andabaNo ratings yet
- FGFHGHJHJKDocument9 pagesFGFHGHJHJKMary Grace VelitarioNo ratings yet
- US4071609Document5 pagesUS4071609Amir RahbariNo ratings yet
- Recovery Method of Nickel From Spent Electroless Nickel Solution by ElectrolyticDocument4 pagesRecovery Method of Nickel From Spent Electroless Nickel Solution by ElectrolyticJonathan Bi NguyenNo ratings yet
- Applied ChemistryDocument10 pagesApplied ChemistryAngelNo ratings yet
- Thionyl Chloride ReactionsDocument7 pagesThionyl Chloride ReactionsMaxim MaximovNo ratings yet
- United States Patent (19) : Hu Et AlDocument4 pagesUnited States Patent (19) : Hu Et AlSepti WidyaNo ratings yet
- Brit. J. Anaesth. (1967), 39, 440: Plant ProcessDocument3 pagesBrit. J. Anaesth. (1967), 39, 440: Plant ProcessPetah LlopNo ratings yet
- Manufacture of Soda Ash - LectDocument10 pagesManufacture of Soda Ash - LectIbrahim Al-MutazNo ratings yet
- Patent Office: United StatesDocument2 pagesPatent Office: United StatesFathia AzzikraNo ratings yet
- Us 2942944Document2 pagesUs 2942944Kristanti NurwidyaningrumNo ratings yet
- Us 4772757Document8 pagesUs 4772757Naomi HerreraNo ratings yet
- Class 10 - Chemistry - Study of Compounds Nitric Acid SolutionsDocument26 pagesClass 10 - Chemistry - Study of Compounds Nitric Acid Solutionsavadhut.malji19No ratings yet
- United States Patent (19) : 75) Inventors: Vlastimil Kadlec, Litvinov VojtéchDocument3 pagesUnited States Patent (19) : 75) Inventors: Vlastimil Kadlec, Litvinov VojtéchGraciaVelitarioNo ratings yet
- Catalyst for Direct Hydration of Ethylene to Ethyl AlcoholDocument3 pagesCatalyst for Direct Hydration of Ethylene to Ethyl AlcoholGraciaVelitarioNo ratings yet
- A01 269Document11 pagesA01 269icingrockNo ratings yet
- Nitric Acid Answer KeyDocument6 pagesNitric Acid Answer KeyGurjapsingh SandhuNo ratings yet
- CP-XVII (Soda Ash & Caustic Soda)Document12 pagesCP-XVII (Soda Ash & Caustic Soda)Usman AliNo ratings yet
- Wilk in 2010Document11 pagesWilk in 2010ulfah nur khikmahNo ratings yet
- Zhang 2014Document5 pagesZhang 2014oviabeautyNo ratings yet
- Alcano 4Document6 pagesAlcano 4Antônio Neto MachadoNo ratings yet
- Driving Equilibria: Dean-Stark Trap: Science Education CollectionDocument2 pagesDriving Equilibria: Dean-Stark Trap: Science Education CollectionLJ RBNo ratings yet
- Question: 3.117 A Motor Draws Electric Power Pelec From A Supply Line ADocument6 pagesQuestion: 3.117 A Motor Draws Electric Power Pelec From A Supply Line AEdilunarNo ratings yet
- Fizik (Ajwad, Eng) PDFDocument122 pagesFizik (Ajwad, Eng) PDFJabar SultanNo ratings yet
- AQA GCSE Chem C3 Practice Question AnswersDocument2 pagesAQA GCSE Chem C3 Practice Question AnswersJawaria MazharNo ratings yet
- Professor Joe Greene Csu, ChicoDocument35 pagesProfessor Joe Greene Csu, ChicoyuxaxaaNo ratings yet
- Heat Transfer: Due Monday 21, JuneDocument5 pagesHeat Transfer: Due Monday 21, JuneAndres TorresNo ratings yet
- Porblem Set 2 (Chapter 8)Document3 pagesPorblem Set 2 (Chapter 8)khozin ltmptNo ratings yet
- McQuarrie Chapter 7 ProblemsDocument7 pagesMcQuarrie Chapter 7 ProblemsUpakarasamy LourderajNo ratings yet
- FCCU OPERATION AND REACTIONS PRESENTATIONDocument44 pagesFCCU OPERATION AND REACTIONS PRESENTATIONHarish GojiyaNo ratings yet
- Lecture 1 Biochemistry 2023-2024Document26 pagesLecture 1 Biochemistry 2023-2024fatimakareem0623No ratings yet
- Use of math in balancing chemical equationsDocument2 pagesUse of math in balancing chemical equationsAshis karmakarNo ratings yet
- Acrylic AcidDocument4 pagesAcrylic AcidDita NainggolanNo ratings yet
- Narayana JeeadvDocument20 pagesNarayana JeeadvsaiNo ratings yet
- LAB REPORT #1 ANALYSISDocument5 pagesLAB REPORT #1 ANALYSISCelive JoyNo ratings yet
- Acrydur 116 ENG US 20 10 2022Document4 pagesAcrydur 116 ENG US 20 10 2022Volkmar LullNo ratings yet
- Trends in The Periodic TableDocument24 pagesTrends in The Periodic TableChris McLeanNo ratings yet
- AnaChem Conjugate Acid Base Pairs 3Document3 pagesAnaChem Conjugate Acid Base Pairs 3Jei HernandezNo ratings yet
- Physics: Summer Vacation HomeworkDocument34 pagesPhysics: Summer Vacation HomeworkTedNo ratings yet
- VOL1Document23 pagesVOL1Tauseef Mansuri100% (1)
- ATMS 305 - Atmospheric Thermodynamics and Statics: - Today's Lecture ObjectivesDocument24 pagesATMS 305 - Atmospheric Thermodynamics and Statics: - Today's Lecture ObjectivesRaul Real GonzalezNo ratings yet
- Ch6 ThermoDocument9 pagesCh6 Thermoyui3456No ratings yet
- Covenant University: SECTION A: Attempt To Answer Question 1 and Any Other Three QuestionsDocument3 pagesCovenant University: SECTION A: Attempt To Answer Question 1 and Any Other Three QuestionsdannyNo ratings yet
- Dr. Shikha Baskar: CHE154: Physical Chemistry For HonorsDocument22 pagesDr. Shikha Baskar: CHE154: Physical Chemistry For HonorsnishitsushantNo ratings yet
- Syllabus (Pharmacy) With Old Version New CreditDocument82 pagesSyllabus (Pharmacy) With Old Version New CreditgitNo ratings yet
- Basic Ideas in ChemistryDocument16 pagesBasic Ideas in ChemistryLucianaAcostaNo ratings yet
- Molecular Orbital Theory and Homonuclear Diatomic Molecules ProblemsDocument5 pagesMolecular Orbital Theory and Homonuclear Diatomic Molecules ProblemsFarid AkhtarNo ratings yet
- Multiphase Flow in PipesDocument23 pagesMultiphase Flow in Pipes000No ratings yet
- Infrared Spectroscopy EceDocument50 pagesInfrared Spectroscopy EceRavi KumarNo ratings yet
- Prevent steam turbine scaling and fouling with water treatmentDocument10 pagesPrevent steam turbine scaling and fouling with water treatmentCarlos CarpioNo ratings yet