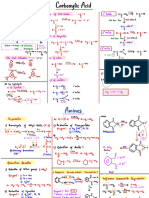
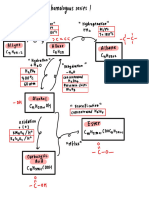
Professional Documents
Culture Documents
Tudou - Fi#Eithciaeephenone: . Och5Ch
Uploaded by
Kratarth YadavOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Tudou - Fi#Eithciaeephenone: . Och5Ch
Uploaded by
Kratarth YadavCopyright:
Available Formats
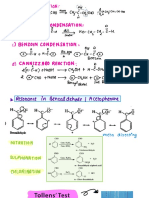
a) ALDOL CONDENSATION :
CHS -
Cfo
+ H CH -
CHO EI Ctg -
no .¥ocH5cH -
- en cao
-
C) CANNIZZARO REACTION
CF Ona
, :
LCH c'IH )
-
+ NaOH → CHGOH t H - -
b) CROSS ALDOL CONDENSATION :
E % I CHO + NaOH → CHIH tf COO Na
- -
% H Benzoate
Ho
cuz Ctf
H Sod
Benzyl alcohol
H
cuz t H
-
- - - - - - -
. .
f) CARBYLAMINE REACTION :
) BALZ SCHIE MANN REACTION :
-
IN H2 "
Nat BE CHzCHgNHg + CH Clg +3 KOH CH
Ctf NEC -13 KCI -13110
-
I
-
fog + ,
NH + CHAZ -13 KOH calc )
Is NIC -13 KCI -13110
fluoro benzene
-
Aniline , .
d) BENZOIN CONDENSATION
g) CLEMMENSEN REDUCTION :
Etat!
:
4GB CH, CHACH, t H2O
Cuzco cuz
In die,
" +
⇐ Even -Ee takin
'
ex - -
f.jo
CHS
+ acid
'
EICH CHS ,
+ Hao
Benzoin .
BRANCH ALLO
BRANCH ALLO
h) DECARBOXYLATION REACTION :
d) FINKELSTEIN REATION :
- 004+2 NaOH ¥fk + Ng cost H2O
GHS -
Br + NAI efA Gtf -
I + Na Br
i) COUPLING REACTION :
Nz tf
-
-
out HC l my f- 17719 REACTION : pm
NaCl + E- on
citing ⇐ Lost> tania "
-
-
K azobenzene
hydroxy
-
-
NaCl t -
NH
, E- Naff -
NH
,
h ) FRIEDA L CRAFT REACTION :
p Amino azobenzene ii)
i) Alkylation Acylation
-
j) DIAZOTIZATION REACTION : Acid " coctlz
← Nitrous
'
NANO
+ HOMO + HCl
,
+ HCl → HOMO + HCl
¥442140
tatsu ¥7 ,
Inc ,
tudou.fi#EItHciAeephenone
O ) GATT ER MANN REACTION :
E- TARD REACTION CHO
cite :%% ÷÷:u
k) : Thu c>
:c PINA
⇐¥
" AE,
.
1- + N
2
Brown complex Benzaldehyde
Toluene
BRANCH ALLO BRANCH ALLO
b) GABRIEL PH THAL IMIDE SYNTHESIS :
'
t) HOFMANN BROM AMIDE DEGRADATION :
NH + KOH
R CON Hg Es
R NH -12 Na Br + , cos -12110 Na
Nk t
GHS III Big +4 NaOH
-
+
'
-
o
- -
,
Phthalimide pot phthalimide E- CONH, +
By -14 KOH Es Et NH, -12K Bztkzcoz -12140
.
IN GHS taiyo EI%: 1- Ca Hs MHz -
u) HUNS DI ECKER REACTION
cc 14
:
Phthalic Acid R COO Agt Bra ¥+7 R Br + cost
Ag Br
phltralimide
- -
N -
Ethyl .
GAITER MANN KOCH REACTION : V) KOLBE 'S REACTION :
g)
¥¥x ¥50 +
"
EI II.97 >
Hcc Na
to + HU co n'Ito
.
Benzaldehyde +
,
>
icgiic Acid )
r) H V. I REACTION C Hell Vol hard
.
Zelinsky Reaction)
Cte, OH tusked UCH OH tu GCU COOH w) REIMER -
TIEMANN REACTION :
, ,
COOH Ck Red P III + cub [ EINE na
.
÷÷Y ,
EIFcucoHL.FI]
Cf C ,
-
s) HOFMANN AMMO NO LYSIS
" amine
:
2.amine
R NH R TR X R
3- amine
R
.
eFcuo ' '"
c salicylate hyde)
H, Ntr X
# R NH TR X ¥ pl
- - -
-
- -
- -
,
R
BRANCH ALLO
X) ROSEN MUND REDUCTION :
a
R - di -
Cl TH, Pd, Bash 's
>
R - d' -
H + HCl ii ) WOLFF KISH NER REDUCTION :
Boiling xylene R CHO-
KN NHL
-
,
KOH
y R.CH, + N,
SAND MEYER REACTION : glycol
y)
del City COCH, CHS Ctf CH
El
"
Caa
fr
- -
→ y ,
+ tea leg
lol
t
+ Hbr ¥ A
Lot
CN - COCH
,
"
t -
CH
,
CHS
+ Ken
iii ) WURTZ REACTION :
E) STEPHEN REDUCTION :
snag -12116 → snag -12cm
R CIN -12cm ) 1- HCl
-
# R CH -
-
-
NH.HU
r÷IIEnIIIII÷r :# Rrr + Imax
I.IM IB
ether
( Hsi:B.ir
-
CH,
Boiling H2O
. .
.
Denker > Cates t2NaBr
4
RCHO tNH4U iv) WURTZ -
FIT TIG REACTION :
i) WILLIAMSON SYNTHESIS CHS Et tanar
,
III.I :B 1¥
-
>
cus
R' ONA ' Toluene
R X -
+ Y ROR + Max .
( Hs I -
t ↳ Hs ONA -
TCH, O -
-
GHS that
ether .
BPANKHALLO
You might also like
- Och5Ch: TakinDocument16 pagesOch5Ch: TakinJohn BushNo ratings yet
- Name Reaction and ConversionDocument11 pagesName Reaction and Conversionwerwer100% (1)
- Carboxyl and Amino QuestionsDocument6 pagesCarboxyl and Amino Questionsatintasya18No ratings yet
- Carboxylic Acid 2Document7 pagesCarboxylic Acid 2bisenpallavi80No ratings yet
- Aldehyde, Ketones and Carboxylic AcidDocument18 pagesAldehyde, Ketones and Carboxylic AcidSimi Sunil100% (2)
- Organic Chemistry Cheat NotesDocument22 pagesOrganic Chemistry Cheat NotesSuryansh SinghNo ratings yet
- Chy-Ch-H: HehehehehehahohDocument2 pagesChy-Ch-H: HehehehehehahohcebrowningNo ratings yet
- HDA Short NotesDocument4 pagesHDA Short Notesadithaj.2006220No ratings yet
- OCOC-III Live Class-1 Teacher NotesDocument43 pagesOCOC-III Live Class-1 Teacher NotesSujal KapoorNo ratings yet
- Practice Question 3Document1 pagePractice Question 3jillNo ratings yet
- Organic Halides Live Class-4 Teacher NotesDocument25 pagesOrganic Halides Live Class-4 Teacher Notessiddhartha singhNo ratings yet
- Tricarboxyllic Acid Cyle (TCA) Krebs Cycle Citric Acid CycleDocument1 pageTricarboxyllic Acid Cyle (TCA) Krebs Cycle Citric Acid CycleSufian MuhammadNo ratings yet
- Adobe Scan Sep 25, 2023Document7 pagesAdobe Scan Sep 25, 2023jvikeshkumar2006No ratings yet
- Alkane, Alkene and Alkyne: Bishwajit PrabhakarDocument185 pagesAlkane, Alkene and Alkyne: Bishwajit PrabhakarAsmit GhoshNo ratings yet
- Imp. Reaction of Aromatic-StructureDocument1 pageImp. Reaction of Aromatic-StructureRoronoa ZoroNo ratings yet
- Organic Chemistry Reactions From MS ChauanDocument3 pagesOrganic Chemistry Reactions From MS ChauanHet GalaNo ratings yet
- Hydrocarbon 2Document27 pagesHydrocarbon 2yashgautam196No ratings yet
- HydrocarbonDocument13 pagesHydrocarbonyashgautam196No ratings yet
- IOC Class-1 NotesDocument21 pagesIOC Class-1 Notesmardarchod 123No ratings yet
- Hydrocarbons Final Revision WorksheetDocument21 pagesHydrocarbons Final Revision WorksheetMoonesh MKNo ratings yet
- H2 Chemistry Organic Reactions Map PracticeDocument1 pageH2 Chemistry Organic Reactions Map PracticeAmami RantaroNo ratings yet
- (Hydrate Carbon) : SugarDocument3 pages(Hydrate Carbon) : Sugarchemistry tutorialNo ratings yet
- BFL F: SteplDocument2 pagesBFL F: SteplNURAISYA QALEEDA BINTI DAMAT / UPMNo ratings yet
- Aldehyde and Ketone HandwrittenDocument2 pagesAldehyde and Ketone HandwrittenHarshvardhan KhatodNo ratings yet
- 19 H.D.A. 15-08-2021 PaperDocument3 pages19 H.D.A. 15-08-2021 PaperArchanaNo ratings yet
- AP Chemistry Basics. - StoichiometryDocument2 pagesAP Chemistry Basics. - StoichiometryAnnabelle WuNo ratings yet
- 30 Most Important Organic ConversionsDocument31 pages30 Most Important Organic ConversionsPiyush GuptaNo ratings yet
- OCOC-1 Live Class-1 Teacher NotesDocument37 pagesOCOC-1 Live Class-1 Teacher Notesmardarchod 123No ratings yet
- Aldehyde, Ketones and Carboxylic AcidDocument18 pagesAldehyde, Ketones and Carboxylic AcidPRADEEP CNo ratings yet
- Organic Chemistry (2) 36 56Document21 pagesOrganic Chemistry (2) 36 56Mr valorant ipadNo ratings yet
- Organic Halides Live Class-7 Teacher NotesDocument36 pagesOrganic Halides Live Class-7 Teacher Notesmardarchod 123No ratings yet
- Carboxylic AcidsDocument23 pagesCarboxylic AcidsSoma Chowdhury RosyNo ratings yet
- CH 3 Acid-BaseDocument7 pagesCH 3 Acid-BaseElle QuizonNo ratings yet
- Alcohols, Phenols, and Ethers Shobhit Nirwan - RemovedDocument9 pagesAlcohols, Phenols, and Ethers Shobhit Nirwan - Removedshoaib1234gkpNo ratings yet
- Alcohols, Phenols, and Ethers Shobhit NirwanDocument10 pagesAlcohols, Phenols, and Ethers Shobhit NirwanKhushi Roy100% (5)
- Alcohols, Phenols, and Ethers Shobhit NirwanDocument9 pagesAlcohols, Phenols, and Ethers Shobhit NirwanMithil MohanrajNo ratings yet
- Dia orDocument8 pagesDia orNaman MahawarNo ratings yet
- Alcohol Phenol Ether (1) 6Document9 pagesAlcohol Phenol Ether (1) 6sdnishacNo ratings yet
- Chemical Engineering JournalDocument14 pagesChemical Engineering JournalWilson UdofiaNo ratings yet
- Obrador2023 SuplementaryDocument5 pagesObrador2023 SuplementaryPilar AufrastoNo ratings yet
- Carbon Compounds NotesDocument3 pagesCarbon Compounds Notesm-8885756No ratings yet
- ETC Part Three Illustration AtfDocument1 pageETC Part Three Illustration Atfpnsscsny29No ratings yet
- Aldehyde Ketones and Carboxylic AcidDocument18 pagesAldehyde Ketones and Carboxylic AcidInfinite SinghNo ratings yet
- 재료의화학적해석 두번째 과제Document2 pages재료의화학적해석 두번째 과제wnrdl2835No ratings yet
- Modul 3Document9 pagesModul 3yuri.angell1234No ratings yet
- TÃ I Liá U Khã NG Ä Æ°á C ShareDocument5 pagesTÃ I Liá U Khã NG Ä Æ°á C ShareHưng Lê NgọcNo ratings yet
- Methylcyolopent-L-Eneq - TT: F It It IlvlightDocument2 pagesMethylcyolopent-L-Eneq - TT: F It It IlvlightJjrlNo ratings yet
- Homework 3Document15 pagesHomework 3Vira TejaNo ratings yet
- Seatwork Preparation and Chemical Reactions of Organic CompoundsDocument6 pagesSeatwork Preparation and Chemical Reactions of Organic CompoundsSo FiaNo ratings yet
- OCOC-II Live Class-1 Teacher NotesDocument41 pagesOCOC-II Live Class-1 Teacher Notesmardarchod 123No ratings yet
- ChemieDocument7 pagesChemieTereza JirouškováNo ratings yet
- Chem 231Document41 pagesChem 231duruNo ratings yet
- Metabolisme ProteinDocument1 pageMetabolisme ProteinuswahNo ratings yet
- Citric Acid Cycle: Step 1 Step 2 Step 8Document3 pagesCitric Acid Cycle: Step 1 Step 2 Step 8Ali Ali AliNo ratings yet
- Not On: UnsymmetrialDocument14 pagesNot On: UnsymmetrialZooper lNo ratings yet
- Homework 1Document7 pagesHomework 1Techno MemerNo ratings yet
- 20 - Metabolismo de PirimidinasDocument1 page20 - Metabolismo de PirimidinasgustasconNo ratings yet
- Alkene PreparationDocument1 pageAlkene Preparationtemp accNo ratings yet
- Organic Chemistry (2) 57 70Document14 pagesOrganic Chemistry (2) 57 70Mr valorant ipadNo ratings yet
- Arjuna JEE 2.0 (2024) : IUPAC NomenclatureDocument3 pagesArjuna JEE 2.0 (2024) : IUPAC NomenclatureDEV SHARMANo ratings yet
- MODULE 4 Alkenes and AlkynesDocument19 pagesMODULE 4 Alkenes and AlkynesSittie Fahieda AloyodanNo ratings yet
- Cellullar RespirationDocument29 pagesCellullar RespirationJohn Lloyd BallaNo ratings yet
- Antioxidants PresentationDocument12 pagesAntioxidants Presentationncjc8591No ratings yet
- Farmakodinamik Dasar Farmakodinamik DasarDocument52 pagesFarmakodinamik Dasar Farmakodinamik DasarRatnaNo ratings yet
- 4-Polyurethanes From Vegetable Oils and Applications A ReviewDocument15 pages4-Polyurethanes From Vegetable Oils and Applications A ReviewSolmazNo ratings yet
- Lechler Paint CatalogueDocument47 pagesLechler Paint CatalogueGeorge WaiteNo ratings yet
- Vista India: Fixed Revised Price-List Month of July-2018Document6 pagesVista India: Fixed Revised Price-List Month of July-2018malay987No ratings yet
- HPLC Columns CosmosilDocument106 pagesHPLC Columns Cosmosilhainguyen2041990No ratings yet
- Assay of Alkaloidal Drugs .Document7 pagesAssay of Alkaloidal Drugs .Imran Gandapur50% (2)
- Characteristics of Carbachol AutosavedDocument5 pagesCharacteristics of Carbachol AutosavedAassh DcmbrNo ratings yet
- Derivados PetroleoDocument48 pagesDerivados PetroleoCarlos TimanaNo ratings yet
- Polymer Synthesis by Enzymatic CatalysisDocument9 pagesPolymer Synthesis by Enzymatic CatalysisIsa AguirreNo ratings yet
- InfoZinzino en MYS SpreadsDocument27 pagesInfoZinzino en MYS SpreadsMia CNo ratings yet
- Review Article The Thiol-Ene (Click) Reaction For The Synthesis of Plant Oil Derived PolymersDocument14 pagesReview Article The Thiol-Ene (Click) Reaction For The Synthesis of Plant Oil Derived PolymersAdnanNo ratings yet
- Polycarbonate Tube Chemical Resistance GuideDocument1 pagePolycarbonate Tube Chemical Resistance GuideInmaNo ratings yet
- An Efficient Heterogeneous Copper Fluorapatite (CuFAP) Catalysed Oxidative Synthesis of Diaryl Sulfone Under Mild Ligand-And Base-Free Conditions PDFDocument5 pagesAn Efficient Heterogeneous Copper Fluorapatite (CuFAP) Catalysed Oxidative Synthesis of Diaryl Sulfone Under Mild Ligand-And Base-Free Conditions PDFjavasoloNo ratings yet
- Solvent: Halaman Utama Profile Images Produk KontakDocument2 pagesSolvent: Halaman Utama Profile Images Produk KontakAndi MursalimNo ratings yet
- D.H. Ripin, D.A. Evans Chem 206: Pka'S of Inorganic and Oxo-AcidsDocument5 pagesD.H. Ripin, D.A. Evans Chem 206: Pka'S of Inorganic and Oxo-AcidsVerónica EspinoNo ratings yet
- Book 1Document4 pagesBook 1Lavya ChadhaNo ratings yet
- Biochemistry Final Review OutlinesDocument22 pagesBiochemistry Final Review Outlineslacey100% (1)
- NO Ingredient Per KG Product SatuanDocument2 pagesNO Ingredient Per KG Product SatuanprishixNo ratings yet
- Apendice 1Document14 pagesApendice 1JA UMAN1No ratings yet
- Biochemistry I ManualDocument16 pagesBiochemistry I ManualKelz mangNo ratings yet
- NyeriDocument1 pageNyeriIlmiyatul MuhimmahNo ratings yet
- Lipids PowerpointDocument15 pagesLipids Powerpointapi-321789351100% (1)
- BIOCHEM - TriacylglycerolDocument7 pagesBIOCHEM - TriacylglycerolChristian AmoloNo ratings yet
- Organometallic Compunds: D. JIM LIVINGSTON, Asst - Prof in Chemistry, ST - John's College, PalaiDocument23 pagesOrganometallic Compunds: D. JIM LIVINGSTON, Asst - Prof in Chemistry, ST - John's College, PalaiJim LivingstonNo ratings yet
- Haloalkane Part I New Syllabus 1.Document16 pagesHaloalkane Part I New Syllabus 1.grgrohit1424No ratings yet
- 1972JAOCS ChemicalCompositionofSunflowerSeedHullsDocument5 pages1972JAOCS ChemicalCompositionofSunflowerSeedHullsMAYETTE PAYABANNo ratings yet