Professional Documents
Culture Documents
Sierra Leone Muslim Congress Secondary School Chemistry Question 2020
Uploaded by
Malike Shamel0 ratings0% found this document useful (0 votes)
11 views2 pagesmalike shamel
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentmalike shamel
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
11 views2 pagesSierra Leone Muslim Congress Secondary School Chemistry Question 2020
Uploaded by
Malike Shamelmalike shamel
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 2
+
4
SIERRA LEONE MUSLIM CONGRESS SECONDARY SCHOOL .
SSI seunmcre na cms
‘Section A (objective)
1, The difference between the mass number of an \sotopesahd its atomic number is:
(2) Directly related to the Identification of element
(b) The number of electrons ‘
(c) The number of neutrons
(d) The number of isotopes
2. Which Theory describes the arrangement and movement of particles in solids, liquid and
Basses?
(a) Theory of relativity (b) Kinetic Theory (c) atomic theory
3. Inwhich state of matter are the particle ‘mostly touching but arranged In a random way?
(a) Solid (b) Liquid (c) Gas
4. What name is given to different versions of atoms that have the same number of proton but
different number of neutron?
(2). Allotropes (b) Twins (c) isotopes (d) isobars
‘5: The formula for sulphuric acid ism H,S0.. How ‘many of each type of atom are there?
(8) Two hydrogen, one sulphur and four oxygen
(b) One hydrogen, two sulohur. and four oxygen
(€) Two hydrogen, two sulphur and four oxygen
6. Inthe Reaction cH, + Or CO; + H,0 what is the formula of a reactant?
= (2) CHy (b) CO, (c) H30
7. What is the meaning of the state symbol (aq)?
(2). Solid (b) dissolved in water (c) gaseous.
8. Which sufi atomic Part of the atom shows the radioactive Properties of the atom
(a) “Blectrons (b) shells (c) protén (d) neutron
9. Which sub-atomic part of the atom talks about the chemical reactivity of the atom?
(2) Electron (b) nucleus (c) proton (d) shells
10. What Is the correct symbol for the element copper? °
{a) CU (b) cu (c)Cu (d) " .
Section B
1a(1) Use Dalton’s theory to explain why potassium nitrate from india or Sierra Leone has the
same mass Percent of K, N, and O,
1a(il)__ why are atom describe as small particle? Explain.
Jalili) what are the postulate of Rutherford and explain with diagram.
1b(i) » Cathode ray tube is Present ini Human being but not present in table. (True or False) and
why? 7
b(i)_Do both members cf the foliowing palrs have the same ‘fumber of protons, Neutrons and
electrons? . .
:
(2) 03!and Og?” (6) Aris and Kis" ce) and Fy!®
1c) what is the Xz notation for each of the atom below?
‘
(a) 18’, 18p*, 20n° (b) 25e", 25p" and. 30n® (c) 47e, 62n° and 4
1c(il) Chlorine has two ean ‘otcurring oe Cand ple if chlorine has an re!
mass of 35, what Is the percentage abundance of each isotopes?
2a(!)__ In the process of balancing the equation.
Al+Cl,—> AICly
Jative atomic
&
Student 1 writes: Al+ Cl, ——> AICI, student 2 writes: Al + Ch—> AICs
Student 3 writes: 2Al +3Cl,—> 2AlCls.
's the approach of the student 1 valid? Student 2? Student 3? Explain.
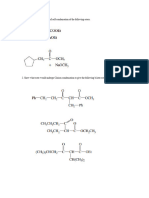
2a(ii)_ write a balanced chemical equations:
Al0;+Fe —> Fe,0,+Al
NHNO, —> NZO—>+H,0
Alx(SO3), + NaOH —> Na,SOs + Al(OH)s
Be,C + H,O—> Be(OH), + CH,
Cu+HNOs —> Cu(NO;), + NO+H,0
KCIO; —> KCI+ 02
o .
2a(il) The relative atomic mass of the element X Is-21.3 and the element is made up, a two
isotopes X** and X°”. What will be the fractional abundance for each Isotope?
2b(i)-- Why are we balancing chemical equation. Explain
2b{ll) classify the following compounds with thelr atomicity ofeach compounds
NaOH. CHsCOOH, C:HsOH, A(OH); ® CuSO, 10H,O Fe,0, Na,SOy
2c(l)__ why do we say the S-Orbitals, P-Orbitals are spherical and dumbbell in shape respectively.
2c(ll) 1s, 2s 2p, 3s 3p. What does the 1, 2, and 3 stands for in term of quantum number?
3a(i) State what rule(S) that is/are Violated or obeyed below: _ ‘
Modell = 15. 3S3P 25 2P
3alil) wh t most common difference between molar mass and Relative molecular mass.
3b(i) ‘calculate the relative molecular mass and the molar mass of the following compounds.
NaSO, AlsPO,, @ Na,CO; 7H,0 . a
3b{ii)” what are the valences of Cu and Fe
3c Identify the radicals below and why
F. CaCl, OH’ and SO,”
4a(i) calculate the percentage composition of oxygen In the compounds below . ze
CasPO, CU20 and Be® (OH), SHO
4a(ii) why Is that the symbol for sodium is not S but Na why.
4b(i) __ what will be the mass of Y in which the X has 70% in, a compound X,Y whose relative ©
% molecular mass |s 150.
“=-4b(II) An Investigation|was made In a compound andit was found that compound have three
Isotopes X** x"*'x"who are In a ratio of 6:4:2, Diaw the graphical form df these (sotopes :
and calculate its relative atomic.mass,
4c why was the symbol for sodium names Na not NA or S. "el
You might also like
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (842)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5807)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1091)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Unit 5 - Acids Bases and Salts Student VersionDocument29 pagesUnit 5 - Acids Bases and Salts Student VersionMalike ShamelNo ratings yet
- Sierra Leone Muslim Congress Chemistry Question 2017Document2 pagesSierra Leone Muslim Congress Chemistry Question 2017Malike ShamelNo ratings yet
- Sierra Leone Muslim Congress Secondary School Chemistry Question 2022Document1 pageSierra Leone Muslim Congress Secondary School Chemistry Question 2022Malike ShamelNo ratings yet
- Sierra Leone Muslim Congress Secondary School Chemistry Question 2013Document2 pagesSierra Leone Muslim Congress Secondary School Chemistry Question 2013Malike ShamelNo ratings yet
- Sierra Leone Muslim Congress Chemistry 2019Document3 pagesSierra Leone Muslim Congress Chemistry 2019Malike ShamelNo ratings yet
- Unit 8 - Solubility and Precipitation Reactions Student VersionDocument19 pagesUnit 8 - Solubility and Precipitation Reactions Student VersionMalike ShamelNo ratings yet
- Sierra Leone Muslim Congress Secondary School Chemistry Question 2022 Sss2Document1 pageSierra Leone Muslim Congress Secondary School Chemistry Question 2022 Sss2Malike ShamelNo ratings yet
- Functional GroupDocument18 pagesFunctional GroupMalike ShamelNo ratings yet
- CHEM 414 Practice Problem SetsDocument4 pagesCHEM 414 Practice Problem SetsMalike ShamelNo ratings yet
- Chem 322 PracticeDocument3 pagesChem 322 PracticeMalike ShamelNo ratings yet
- Production of Biofuel Mini Project PreseDocument18 pagesProduction of Biofuel Mini Project PreseMalike ShamelNo ratings yet
- Polymer Solutions2a Lecture 2 Print VRSNDocument11 pagesPolymer Solutions2a Lecture 2 Print VRSNMalike ShamelNo ratings yet