Professional Documents
Culture Documents
Sierra Leone Muslim Congress Secondary School Chemistry Question 2013
Sierra Leone Muslim Congress Secondary School Chemistry Question 2013
Uploaded by
Malike Shamel0 ratings0% found this document useful (0 votes)
14 views2 pagesMr Lamin Sankoh myquizy.com
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentMr Lamin Sankoh myquizy.com
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
14 views2 pagesSierra Leone Muslim Congress Secondary School Chemistry Question 2013
Sierra Leone Muslim Congress Secondary School Chemistry Question 2013
Uploaded by
Malike ShamelMr Lamin Sankoh myquizy.com
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 2
oi :
i SIERRA LEONE MUSLIM CONGRESS SENIOR SECONDARY SCHOOL,
IS5Y MESS-MESS FREETOWN
FIRST TERM EXAMINATION — 2013. “ ¥
Mi CPIME: 1% hrs;
‘er four questions in all — question (1) one and any other three (3)
ion A s :
QI. (a) Copy and complete the table below
Inference
*TA white precipitate which ts
TNO seg
| Add AgNa)s (oq) ta
substance followed by
| dilute HNO and excess
LNEh. ——-4— |
| Add dilute HCE with som :
[som solid substance COs" present
(b) Give 7 (5) examples of} (soluble salts ~ (II) Inséluble salts
(©) (i) What are acid-base indicators? (ii) Give three examples of indicators you
have studied
(a) Copy and complete the table below:
CE™ present
fete NHOHay |
[ Strong acid V5 weak
{base pees
| Weak acid V5 strong
carbonate or hydrogen |’ Methyl Orange
carbonate
Section B ~ (Answer any three (3) questions
Q2. (a) Whot is meant by Polar Covatency? oa
(i) Give two factors that influence covalent bonding. e
(ii) Give three (3) characteristics of electrovalent
(b) Gi) Define the term pH.
(1) Ap analysis showed that iuman bleod has a ptt of 740, Calculate the [Hof human ie
blood.
(c) (i) Consider the figures as illustrated below
Liquid ‘e
Ta Xe iy S :
x ‘ ey ee
Gas «Solid. » eo ate
R 4 ee Bl G :
». State the processes represented by P, Q and
(ii) Some solids when heated changes direetly tof
ef (1). *What name is given to this phenomenon? ~~ _. wn
* (11) Name two substances which exhibit the phenemenon above
(ii) State nwo differences between boiling ard evaporation, (25 marks)"
Me ey
BS
\
¥
Q3. (a) State Graham's law of diffusion
(b) When 28.0cm! of oxygen gas at 27°C and a constant pressure was immersed in an ice
bath at 0°C, the volume of oxygen decreased, .
(i) Which gas law is illustrated by ply oscrvations? (ii) State the gas law
(iii) Sketch a graph'to illustrate this law (iv) Calcylate the new volume of oxygen.
. (©) Give two (2) examples of each of the following: i
(1) Alkali (11) Acid salt (III) Acidic oxides (IV) Docile salt’ (V) Amphotenic oxide
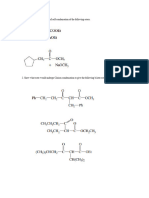
(4) Give the IUPAC name of each of the follwing substance.
(OKA (CN)6 (I) CuSO4 2120 (11) FAL (S04) 2, 1210. (25 marks)
Q4. (a) Whot are salts?
(b) A given fixed mass ofa aSoccupies 20cm? at YECAnd 1.84 x 10'Nm? pressures.
Calculate the volume of the gas at s.t Pp 7
Standard temp = 273k
Standard presbire = 1.01 x rosie ’
+) @) State the type of bond that ex... * cach of the following substance
()MgO — (ID.NH? (IH) Fe (IV) HF
(i) Exptain why alcohols conapletely dissolves in water.
(@) Classify each of the following solutions as acidic basic or neutral
, G) NICE (oq, (ii) K2COs ) Gli) AICES” (iv) CH C00 Na nq) (25 marks)
05. (a) Detine the following: (i) Deliquescence suostance (ii) Ilygrose~pic substance
(©) 4 solution containsV.00006 mol/ of NaOH solittion. Determine the
C(O] GH) Giiy pH iv) pott
(¢) State two chemical properties of acid =
(d) Give three (3) ex mples of gach of the, following!, §—— ——
. ,, (Drying agent Gi)deliquescent . . (iiyeMorgécence (iv) Hydrated salts
‘ 1 sy) teid anhydride tine -
: We Od 7 Me $043
= awe SS RB
Re ay
<=
You might also like
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (842)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5807)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1091)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (346)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Sierra Leone Muslim Congress Chemistry 2019Document3 pagesSierra Leone Muslim Congress Chemistry 2019Malike ShamelNo ratings yet
- Sierra Leone Muslim Congress Secondary School Chemistry Question 2022Document1 pageSierra Leone Muslim Congress Secondary School Chemistry Question 2022Malike ShamelNo ratings yet
- Sierra Leone Muslim Congress Chemistry Question 2017Document2 pagesSierra Leone Muslim Congress Chemistry Question 2017Malike ShamelNo ratings yet
- Sierra Leone Muslim Congress Secondary School Chemistry Question 2020Document2 pagesSierra Leone Muslim Congress Secondary School Chemistry Question 2020Malike ShamelNo ratings yet
- Sierra Leone Muslim Congress Chemistry QuestionDocument2 pagesSierra Leone Muslim Congress Chemistry QuestionMalike ShamelNo ratings yet
- Sierra Leone Muslim Congress Secondary School Chemistry Question 2022 Sss2Document1 pageSierra Leone Muslim Congress Secondary School Chemistry Question 2022 Sss2Malike ShamelNo ratings yet
- Unit 5 - Acids Bases and Salts Student VersionDocument29 pagesUnit 5 - Acids Bases and Salts Student VersionMalike ShamelNo ratings yet
- Functional GroupDocument18 pagesFunctional GroupMalike ShamelNo ratings yet
- Unit 8 - Solubility and Precipitation Reactions Student VersionDocument19 pagesUnit 8 - Solubility and Precipitation Reactions Student VersionMalike ShamelNo ratings yet
- CHEM 414 Practice Problem SetsDocument4 pagesCHEM 414 Practice Problem SetsMalike ShamelNo ratings yet
- Polymer Solutions2a Lecture 2 Print VRSNDocument11 pagesPolymer Solutions2a Lecture 2 Print VRSNMalike ShamelNo ratings yet
- Chem 322 PracticeDocument3 pagesChem 322 PracticeMalike ShamelNo ratings yet
- Production of Biofuel Mini Project PreseDocument18 pagesProduction of Biofuel Mini Project PreseMalike ShamelNo ratings yet