Professional Documents
Culture Documents
Sierra Leone Muslim Congress Chemistry Question 2017
Sierra Leone Muslim Congress Chemistry Question 2017
Uploaded by
Malike Shamel0 ratings0% found this document useful (0 votes)
6 views2 pagesMalike Shamel myquizy.com
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentMalike Shamel myquizy.com
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
6 views2 pagesSierra Leone Muslim Congress Chemistry Question 2017
Sierra Leone Muslim Congress Chemistry Question 2017
Uploaded by
Malike ShamelMalike Shamel myquizy.com
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 2
NDARY SCHOOL
2
SECOND TERM EXAMINATION MARCH201
oy’ OST STRY SSS J - TIME. 2 hours
EMIS
SECTION A — Answer all questions in this section (iv) Radical
QI. (a) Define the following: (i) Matter (ii) Compound “ Mixtae,
(b) Name the method used to separate each of the following mixture:
(i) Kerosene and water (ji) Ammonium chloride and Sodium ¢]
ii) Petroleum (iv) Dyes in a black ink
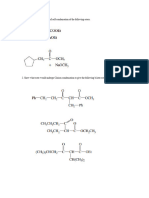
(©) Give the IUPAC names of the follo\ ing subetences '
(NayCrO, (ii) KMn0y (iii) CaCoy . The third
(d) An organic liquid A was found to contain 60% carbon and 13.3% Lanes ‘qubetarico
‘component was assumed to be oxygen. The relative molecular mass of the Su
was found to be 60, Calculate the empirical formula and molecular formula
compound A a
(©) Distinguish between miscible and immiscfble liquid » : 7
(® Determine the oxidation state of carbon in each of the following compoun
2
(CO, (i)CO, — (iii) CO_(iv) Na2CO3
(g) Write the S.P.D.E notation of the following species
@Ca (ii) AL (iii) CI_— (iv) Ne
(h) State two differences between mixture and compound XN
(i) Determine the valences of the following atoms: (a)Li (b)O ()Ar (4) Na .
@ Write the chemical formulae of the following compound. id
i) Aluminum Oxide (ii) Iron (ii) Oxide Calcium Oxide (iv) Hydrogen Oxide |
SECTION B — Answer any two questions ° ’
Q2. (@) Define the following terms: (1) Isotopes _ (ji) Allotropes
(b) PCL and °7c =
(i) Name the phenomenon illustrated above
Gi) Why the two atoms have different mass nuiber name two other element that can
exhibits this same phenomeno’
(@) The empirical formula of a compound is given as CHO and relative molecular mass
is 60. Calculate the molecular formula of the compound.
§ Q3. (@) Give the correct meaning of the following Abbreviations: (i) IUPAC (II) $.P.D.F
(b) Using the $.P.D.F notation, write down the electronic configuration of the following
substances: (i) Zn (ii) Cu (ili). Cu*— (ivy $?
J (©) Copy and.complete the'table below 2
Particle | Number of | Number of | Number of. | Mas
Proton | Electron _§| Neutron’ 2s eee ae
QA. (>The diagram below indicates a sét Up of certain experiment:
x pr |
ee
Vv
‘Using the diagram above answer the following questior
(i) Suggest a title for the experiment (ii) name the labeled parts I ms II important?
(iii) Name two mixtures that can separate using this method (iv) why i
(b) With a reason classify the following elements into metals and non metals
(i) Sulphur (i) oxygen (iii) magnesium (iv) potassium
(c) Determine the oxidation states of nitrogen in the following compounds:
(@)NO — (b)NOz_ (©)N203_ (4) N20
QS. (@) Explain the term distillation by the
(b) calculate the relative molecular mass of the following compound as represented by
formular :
@)NO2CO; (ii) Fex(SO4)3_ (iii) (NH) POs (iv) Cu(OH)2
(Given that: Na=23, C=12 0-16, $=32, Cu=63.5.Fe = 56]
(©) Name two magnetic substances and three non-magnetic substances .
You might also like
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (842)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5807)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1091)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (346)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Sierra Leone Muslim Congress Secondary School Chemistry Question 2020Document2 pagesSierra Leone Muslim Congress Secondary School Chemistry Question 2020Malike ShamelNo ratings yet
- Sierra Leone Muslim Congress Chemistry 2019Document3 pagesSierra Leone Muslim Congress Chemistry 2019Malike ShamelNo ratings yet
- Sierra Leone Muslim Congress Secondary School Chemistry Question 2022Document1 pageSierra Leone Muslim Congress Secondary School Chemistry Question 2022Malike ShamelNo ratings yet
- Sierra Leone Muslim Congress Secondary School Chemistry Question 2013Document2 pagesSierra Leone Muslim Congress Secondary School Chemistry Question 2013Malike ShamelNo ratings yet
- Sierra Leone Muslim Congress Chemistry QuestionDocument2 pagesSierra Leone Muslim Congress Chemistry QuestionMalike ShamelNo ratings yet
- Sierra Leone Muslim Congress Secondary School Chemistry Question 2022 Sss2Document1 pageSierra Leone Muslim Congress Secondary School Chemistry Question 2022 Sss2Malike ShamelNo ratings yet
- Unit 5 - Acids Bases and Salts Student VersionDocument29 pagesUnit 5 - Acids Bases and Salts Student VersionMalike ShamelNo ratings yet
- Functional GroupDocument18 pagesFunctional GroupMalike ShamelNo ratings yet
- Unit 8 - Solubility and Precipitation Reactions Student VersionDocument19 pagesUnit 8 - Solubility and Precipitation Reactions Student VersionMalike ShamelNo ratings yet
- CHEM 414 Practice Problem SetsDocument4 pagesCHEM 414 Practice Problem SetsMalike ShamelNo ratings yet
- Polymer Solutions2a Lecture 2 Print VRSNDocument11 pagesPolymer Solutions2a Lecture 2 Print VRSNMalike ShamelNo ratings yet
- Chem 322 PracticeDocument3 pagesChem 322 PracticeMalike ShamelNo ratings yet
- Production of Biofuel Mini Project PreseDocument18 pagesProduction of Biofuel Mini Project PreseMalike ShamelNo ratings yet