Professional Documents
Culture Documents
Sierra Leone Muslim Congress Chemistry Question
Sierra Leone Muslim Congress Chemistry Question
Uploaded by
Malike Shamel0 ratings0% found this document useful (0 votes)
11 views2 pagesmyquizy.com
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentmyquizy.com
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
11 views2 pagesSierra Leone Muslim Congress Chemistry Question
Sierra Leone Muslim Congress Chemistry Question
Uploaded by
Malike Shamelmyquizy.com
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 2
Section B = answer any two questions (70 marks)
|. (a) (i) Detine the following: (1) Empirical formula
. (ID) Molecular formula (2 marks)
(ii) A hydrocarbon X, contains 88.9%
the molecular formula of the com
(b) (i) Define radioactivity, (2 marks)
(it) Give four (4) characteristics of gamma radiation
(iii) Complete the nuclear equation below:
a
14C ———+» 14N4x (3 marks)
6 7
(e) (i) State the periodic law
(ii) Give two examples of cach family
(1) Alkaline metals (2 marks)
(II) Alkaline earth metals (2 marks)
(Ul) Halogen (2 marks)
(IY) Noble gases (2 marks)
(d) In an experiment, it was found that 3.0;
Produce carbondioxide as shown in the equation below:
— Cu) +O —> C024 calculate the:
()) "Mass of oxygen required to bun 3.0g of carbon
(ii) ‘volume of carbondioxide Produce at S.t.p when 3.0g
(2 marks)
» carbon and has a vapour
pound. [C = 12.0, H = 1.0]
excess Oxygen’ (5 marks)
[C= 12,)=.16, molar volume o
2. (a) () Define the fotlowing: (i) Standard Solution (ii) Molar s
(it) A solution was made 10 contain 50cm? of 0:42M NaOH. (
(i) Nuthber of moles of NaOH - ® Mass of Ni
: gl NaOH = 40g mol ~!] (6 marks)
(b) (i) Whatvotunte of water must be added toT50cin! of 4,5
f gas at s:t.p 22.4dm?]
glu
]aOH present in
IMINO; to makes
exactly 1.5M2-) (3marks) v *
(ii) Give five (5) periodic Properties that
(iii) How is the term Period and
(©) (i) Give reasons for th
(i) Atomi
you have studied (3m
group relate to the periddic table
ie following observations
izes of element de
(2 marks)” .
ce. Calculate they
cm” of the substan
(i) Concentration of Na2CO3 ing dm— 3
Concentration of Na2C03 in mol dm ~3
ENa2C03 = 106g mol! } (marks)
“3. @(@ Define the following:” (i) Atomic size
* Gi) 1,458 ofa 8ascousthydrocarbon Q gontai
(i) f atbon toms
(ii) Ionization. Engrg
f are present on this mas:
H of hydrogen atoms are present in th
Caleatite the empirical formula of.
f the relative ‘molar mass ofQ ig 8:
° T= 1Lo'er 12,0) ° R29
' :
MD Gar, > G-dunyors
ON GL Yge
‘ ‘
8 of carbon burns with excess ox
“alculate thd
Ms 1.22 of carbon and 0:2:
Sc yer
OL (8 marks) MSL moleguly ymuian
density 27. Ci
(6 marks)
of carl
ition (4
the solut,
(4 marks)
Sg of hydrogen
of :
S
vid
sof radiations G marks)
Jements (3 marks)
tion metals or theit oxi
(b) @ Give the name and nature of the type:
( ii) Give five (5) examples of transition ¢
(iii) Give three (3) examples of any transi
catalyst. (3 marks)
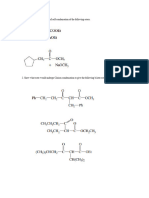
(c) (i) Write a balance chemical equations to show the reaction of chlorine with
(ii) Dilute alkali (6 marks)
ides used as
(i) Hot concentrated alkali
(ii) Give three (3) physical properties of the Group VIILA clements (Halogens)
(3 marks)
(d) (i) Determine the molar mass of each of the following compound
(i) NaxC03.10H;0 (ii) KMnOx (iii) Cis His Coo (iv) CH, CooC2Hs
Na = 23, C = 12, K =39, H= 1, ) = 16, Mn = 56
(ii) Give four (4) uses of radioactivity
GOOD LUCK!!! .
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5819)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1092)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (845)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (348)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (822)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Sierra Leone Muslim Congress Chemistry Question 2017Document2 pagesSierra Leone Muslim Congress Chemistry Question 2017Malike ShamelNo ratings yet
- Sierra Leone Muslim Congress Secondary School Chemistry Question 2022Document1 pageSierra Leone Muslim Congress Secondary School Chemistry Question 2022Malike ShamelNo ratings yet
- Sierra Leone Muslim Congress Chemistry 2019Document3 pagesSierra Leone Muslim Congress Chemistry 2019Malike ShamelNo ratings yet
- Sierra Leone Muslim Congress Secondary School Chemistry Question 2020Document2 pagesSierra Leone Muslim Congress Secondary School Chemistry Question 2020Malike ShamelNo ratings yet
- Sierra Leone Muslim Congress Secondary School Chemistry Question 2013Document2 pagesSierra Leone Muslim Congress Secondary School Chemistry Question 2013Malike ShamelNo ratings yet
- Sierra Leone Muslim Congress Secondary School Chemistry Question 2022 Sss2Document1 pageSierra Leone Muslim Congress Secondary School Chemistry Question 2022 Sss2Malike ShamelNo ratings yet
- Functional GroupDocument18 pagesFunctional GroupMalike ShamelNo ratings yet
- Unit 8 - Solubility and Precipitation Reactions Student VersionDocument19 pagesUnit 8 - Solubility and Precipitation Reactions Student VersionMalike ShamelNo ratings yet
- Unit 5 - Acids Bases and Salts Student VersionDocument29 pagesUnit 5 - Acids Bases and Salts Student VersionMalike ShamelNo ratings yet
- Polymer Solutions2a Lecture 2 Print VRSNDocument11 pagesPolymer Solutions2a Lecture 2 Print VRSNMalike ShamelNo ratings yet
- Chem 322 PracticeDocument3 pagesChem 322 PracticeMalike ShamelNo ratings yet
- Production of Biofuel Mini Project PreseDocument18 pagesProduction of Biofuel Mini Project PreseMalike ShamelNo ratings yet
- CHEM 414 Practice Problem SetsDocument4 pagesCHEM 414 Practice Problem SetsMalike ShamelNo ratings yet