Professional Documents
Culture Documents
Xi CRP Iit Che CPT QP 14.01.2024
Uploaded by
Deena chemist0 ratings0% found this document useful (0 votes)
6 views2 pagesThis document contains 50 multiple choice questions related to chemistry concepts like alkanes, alkenes, aromatic compounds, isomers, and chemical reactions. The questions cover topics such as identifying alkane structures, ordering boiling points of pentane isomers, predicting products of reactions involving sodium, alkene additions and substitutions, aromatic conversions, and calculating bond properties.
Original Description:
Original Title
XI CRP IIT CHE CPT QP 14.01.2024
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document contains 50 multiple choice questions related to chemistry concepts like alkanes, alkenes, aromatic compounds, isomers, and chemical reactions. The questions cover topics such as identifying alkane structures, ordering boiling points of pentane isomers, predicting products of reactions involving sodium, alkene additions and substitutions, aromatic conversions, and calculating bond properties.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
6 views2 pagesXi CRP Iit Che CPT QP 14.01.2024
Uploaded by
Deena chemistThis document contains 50 multiple choice questions related to chemistry concepts like alkanes, alkenes, aromatic compounds, isomers, and chemical reactions. The questions cover topics such as identifying alkane structures, ordering boiling points of pentane isomers, predicting products of reactions involving sodium, alkene additions and substitutions, aromatic conversions, and calculating bond properties.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
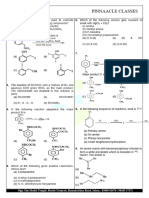
CHEMISTRY
26. Which represents an alkane
(a) 𝐶5 𝐻8 (b) 𝐶8 𝐻6 (c) 𝐶9 𝐻10 (d) 𝐶7 𝐻16
27. The decreasing order of boiling points is
(a) n-Pentane > iso-Pentane > neo-Pentane
(b) iso-Pentane > n-Pentane > neo-Pentane
(c) neo-Pentane > iso-Pentane > n-Pentane
(d) n-Pentane > neo-Pentane > iso-Pentane
28. To prepare a pure sample of n-hexane using sodium metal as one reactant,
the other reactant will be
(a) n-propyl bromide
(b) Ethyl bromide and n-butyl bromide
(c) Ethyl chloride and n-butyl chloride
(d) Methyl bromide and n -pentyl chloride
29. Sodium acetate can be converted to ethane by
(a) Heating with 𝐿𝑖𝐴𝑙𝐻4 (b) Electrolysing its aqueous solution
(c) Heating with soda lime (d) Heating with calcium acetate
30. In the reaction 𝐶𝐻3 − 𝐵𝑟 + 2𝑁𝑎 + 𝐵𝑟 − 𝐶𝐻3 →, the product called
(a) Wurtz reaction (b) Aldol condensation
(c) Perkin’s reaction (d) Levit reaction
31. Iodoethane reacts with sodium in the presence of dry ether. The product is
(a) Pentane (b) Propane (c) Butene (d) Butane
32. Which of the following is oxidised by 𝐾𝑀𝑛𝑂4
(a) Methane (b) Pentane (c) Isobutane (d) Neopentane
33. When ethylene bromide is treated with 𝑍𝑛, we get
(a) Alkane (b) Alkene (c) Alkyne (d) All
34. Ethene when treated with 𝐵𝑟2 in the presence of 𝐶𝐶𝑙4 which compound is formed
(a) 1, 2-dibromoethane (b) 1-bromo-2-chloroethane
(c) Both (a) and (b) (d) 1, 1, 1-tribromoethane
35. Alkenes usually show which type of reaction
(a) Addition (b) Substitution (c) Elimination (d) Superposition
36. The propene reacts with HBr to form
(a) Ethane (b) Hexane (c) 1-bromo-propane (d) 2-bromo propane
37. Ethylene may be obtained by dehydration of which of the following with
concentrated 𝐻2 𝑆𝑂4 at 160 − 170𝑜 𝐶
(a) 𝐶2 𝐻5 𝑂𝐻 (b) 𝐶𝐻3 𝑂𝐻 (c) 𝐶𝐻3 𝐶𝐻2 𝐶𝐻2 𝑂𝐻 (d) (𝐶𝐻3 )2 𝐶𝐻𝐶𝐻2 𝑂𝐻
38. The disappearance of the characteristic purple colour of 𝐾𝑀𝑛𝑂4 in its reaction with
an alkene is the test for unsaturation. It is known as
(a) Markownikoff's test (b) Baeyer's test
(c) Wurtz's test (d) Grignard test
39. A gas formed by the action of alcoholic KOH on ethyl iodide, decolourises
alkaline𝐾𝑀𝑛𝑂4 . The gas is
(a) 𝐶2 𝐻6 (b) 𝐶𝐻4 (c) 𝐶2 𝐻2 (d) 𝐶2 𝐻4
anhydrous
40. 𝐶6 𝐻6 + 𝐶𝐻3 𝐶𝑙 → 𝐶6 𝐻5 𝐶𝐻3 + 𝐻𝐶𝑙 is an example of
𝐴𝑙𝐶𝑙3
(a) Friedel-Craft's reaction (b) Kolbe's synthesis
(c) Wurtz reaction (d) Grignard reaction
41. On heating a mixture of sodium benzoate and sodalime, the following is obtained
(a) Toluene (b) Phenol (c) Benzene (d) Benzoic acid
42. Benzene on treatment with a mixture of conc. 𝐻𝑁𝑂3 and conc. 𝐻2 𝑆𝑂4 at 100𝑜 𝐶 gives
(a) Nitrobenzene (b) m-dinitrobenzene
(c) p-dinitrobenzene (d) o-dinitrobenzene
43. Aromatisation of n-heptane by passing over (𝐴𝑙2 𝑂3 + 𝐶𝑟2 𝑂3 ) catalyst at 773 K gives
(a) Benzene (b) Toluene (c) Mixture of both (d) Heptylene
44. Which among the following is anti-aromatic ?
(a) (b) (c) (d)
45. Which of the following molecules have all C–C bonds are of equal length?
–
(a) (b) (c) (d) All of these
46. Total resonance structure of phenol ________.
47. Number hybrid orbital in ethane ________.
48. Number pure orbital in benzene _______.
49. Double bond equivalence of benzene ______.
50. Sum of 𝜋 and 𝜎 bond in 1,3 butadiene ______.
You might also like
- Raffia Tape Line ProcessDocument20 pagesRaffia Tape Line ProcessSumeet Rathor100% (12)
- Xi Rasi Neet Che WPT QP 22.01.2024Document3 pagesXi Rasi Neet Che WPT QP 22.01.2024Deena chemistNo ratings yet
- Xi CRPDocument3 pagesXi CRPDeena chemistNo ratings yet
- Xi CRP Neet Che WPT QP 31.12.2023Document3 pagesXi CRP Neet Che WPT QP 31.12.2023Deena chemistNo ratings yet
- Alkanes 24.12...........Document4 pagesAlkanes 24.12...........vengateshwaran kNo ratings yet
- Chemistry Ch9,10 Part IIDocument4 pagesChemistry Ch9,10 Part IIdania.siddiqui195No ratings yet
- Aromatic Hydrocarbon (Q.B.) (MSC)Document14 pagesAromatic Hydrocarbon (Q.B.) (MSC)Raj ModiNo ratings yet
- 12th Chemistry CH-16MCQsDocument3 pages12th Chemistry CH-16MCQsM. ABDUR REHMANNo ratings yet
- Alcohol's, Phenols & Ethers (MCQ'S)Document2 pagesAlcohol's, Phenols & Ethers (MCQ'S)PATEL AUM S.No ratings yet
- SHREE Class Islampur Alkanes) : Chemistry (MHT-CET 2021Document3 pagesSHREE Class Islampur Alkanes) : Chemistry (MHT-CET 2021Archana MoreNo ratings yet
- 12th Chemistry CH-15MCQsDocument4 pages12th Chemistry CH-15MCQsRana DugNo ratings yet
- Alcohols, Phenols MCQDocument13 pagesAlcohols, Phenols MCQSnekha TNo ratings yet
- Chemistry-FUNGAT/ECAT: (Chapter 7+8+9 B-II)Document2 pagesChemistry-FUNGAT/ECAT: (Chapter 7+8+9 B-II)XXXNo ratings yet
- HaloDocument17 pagesHaloadityakatariya157No ratings yet
- Nsec 1999Document12 pagesNsec 1999CorneliaNo ratings yet
- Organic Compounds Containing NitrogenDocument6 pagesOrganic Compounds Containing Nitrogenkavitha2511977No ratings yet
- DPT-17 Chem & Zoo Neet 21.01.24Document12 pagesDPT-17 Chem & Zoo Neet 21.01.24pinnaacleclasses salemNo ratings yet
- NEET - Haloalkanes & Haloarenes - (Q+S)Document18 pagesNEET - Haloalkanes & Haloarenes - (Q+S)Sachin DedhiaNo ratings yet
- MCQ S-1Document8 pagesMCQ S-1kavisanjurohillaNo ratings yet
- 5358chemistry Class XII Question Bank (First Part) (2022-23)Document27 pages5358chemistry Class XII Question Bank (First Part) (2022-23)Jiya PandeyNo ratings yet
- DPP Alkanes2Document4 pagesDPP Alkanes2Vinod AgrawalNo ratings yet
- Organic Chemistry Questions2023Document11 pagesOrganic Chemistry Questions2023xqfs2cd44sNo ratings yet
- Hydrocarbons Q 2Document3 pagesHydrocarbons Q 2REJA MUKIB KHANNo ratings yet
- Chem Class 12 WorksheetDocument8 pagesChem Class 12 WorksheetBHAVYA KUSHWAHANo ratings yet
- AIIMS 2019 Chemistry Sample Question PaperDocument10 pagesAIIMS 2019 Chemistry Sample Question PapermisostudyNo ratings yet
- Alkyl HalideDocument8 pagesAlkyl HalideMegh Raj BhattNo ratings yet
- PG Organic Unit - IVDocument10 pagesPG Organic Unit - IVElakkiya shankarNo ratings yet
- CH-12 - MCQS Ald, Ket & Car - AcidsDocument3 pagesCH-12 - MCQS Ald, Ket & Car - AcidsPranav ShankarNo ratings yet
- DPT-48 Chem & Zoo Neet 01.03.24Document13 pagesDPT-48 Chem & Zoo Neet 01.03.24pinnaacleclasses salemNo ratings yet
- Aromatic Compound SheetDocument71 pagesAromatic Compound Sheetadityavaish739No ratings yet
- Aldehydes, Ketones and Carboxylic AcidsDocument5 pagesAldehydes, Ketones and Carboxylic AcidsShazia FarheenNo ratings yet
- Alcohols and PhenolsDocument9 pagesAlcohols and Phenolsdivya divyaNo ratings yet
- GENERAL ORGANIC CHEMISTRY 60 QuestionsDocument67 pagesGENERAL ORGANIC CHEMISTRY 60 Questionssradhasreeni68No ratings yet
- C11 - ALCOHOLS PHENOLS & ETHERS (1) .9c6f83eDocument4 pagesC11 - ALCOHOLS PHENOLS & ETHERS (1) .9c6f83eakashkishore363No ratings yet
- Carbonyl Compounds 12thDocument24 pagesCarbonyl Compounds 12thRaju SinghNo ratings yet
- Monthly Test Class - Xii Subject - ChemistryDocument10 pagesMonthly Test Class - Xii Subject - ChemistryHîмanî JayasNo ratings yet
- Dec 2013Document90 pagesDec 2013sridharR hahahaNo ratings yet
- Chemistry Question-HydrocarbonDocument4 pagesChemistry Question-Hydrocarbonpogboi2342No ratings yet
- Alkyl and Aryl Halide TestDocument6 pagesAlkyl and Aryl Halide TestSoren Sharma50% (6)
- Practice Test Chemistry CL 12Document10 pagesPractice Test Chemistry CL 12Coopin loopNo ratings yet
- 12.Mcq - Aldehydes Ketones Carboxylic AcidsDocument23 pages12.Mcq - Aldehydes Ketones Carboxylic AcidsBedosi Bidita PandaNo ratings yet
- JEE - Haloalkanes & Haloarenes - (Q+S)Document13 pagesJEE - Haloalkanes & Haloarenes - (Q+S)Sachin DedhiaNo ratings yet
- Hydrocarbon - Practice SheetDocument3 pagesHydrocarbon - Practice SheetAbhishek PathakNo ratings yet
- Alkanes - Alkenes - Alkynes - DPP 3Document3 pagesAlkanes - Alkenes - Alkynes - DPP 3Vishal_93100% (1)
- Haloalkanes and HaloarenesDocument5 pagesHaloalkanes and Haloareneskavitha2511977No ratings yet
- Organic SolveDocument6 pagesOrganic SolveKR KhanNo ratings yet
- Nsec Paper Discussion NotesDocument63 pagesNsec Paper Discussion NotesMamata JalendraNo ratings yet
- Chem CGRDocument5 pagesChem CGRpinnaacleclasses salemNo ratings yet
- Answer: CDocument15 pagesAnswer: CHarryNo ratings yet
- AliphaticDocument3 pagesAliphaticAdel AliNo ratings yet
- Aromatic Compounds (13th)Document24 pagesAromatic Compounds (13th)Raju SinghNo ratings yet
- Alkyne and AromaticDocument3 pagesAlkyne and AromaticJayanth KNo ratings yet
- Chemistry Mcqs by KashuDocument27 pagesChemistry Mcqs by KashuZulfqar AhmadNo ratings yet
- NEET - Halo Alkanes and Halo Arenes Practice PaperDocument3 pagesNEET - Halo Alkanes and Halo Arenes Practice PaperGanga DharaNo ratings yet
- Hydrocar SHEET3Document4 pagesHydrocar SHEET3Aayush SaxenaNo ratings yet
- Chemistry 2011: (A) 0 G (B) 0.125g (C) 1g (D) 0.5gDocument6 pagesChemistry 2011: (A) 0 G (B) 0.125g (C) 1g (D) 0.5gVivek SharmaNo ratings yet
- 12TH CBSE DPP 37. Aldehydes, Ketones and Carboxylic Acids MCQ ASSERTION REASON CS QDocument20 pages12TH CBSE DPP 37. Aldehydes, Ketones and Carboxylic Acids MCQ ASSERTION REASON CS Q123No ratings yet
- Chem 12 Term 1Document5 pagesChem 12 Term 1shikhajha9b33No ratings yet
- Lecturer Chemistry Mcqs PSC Past PaperDocument28 pagesLecturer Chemistry Mcqs PSC Past PaperNauman Khalid0% (1)
- The Total Synthesis of Natural ProductsFrom EverandThe Total Synthesis of Natural ProductsJohn ApSimonNo ratings yet
- Xii DPT Bot 29.03.24Document6 pagesXii DPT Bot 29.03.24Deena chemistNo ratings yet
- WPT Xi Rasi Che Neet Key 2-12-23Document2 pagesWPT Xi Rasi Che Neet Key 2-12-23Deena chemistNo ratings yet
- WPT Xi Centre Che Neet Key 10-12-23Document3 pagesWPT Xi Centre Che Neet Key 10-12-23Deena chemistNo ratings yet
- DPT 33 Centre Rasi Iit Jee Che Key 09-12-23Document4 pagesDPT 33 Centre Rasi Iit Jee Che Key 09-12-23Deena chemistNo ratings yet
- DPT 31 Xii Centre Rasi Che Neet Key 07-12-23Document8 pagesDPT 31 Xii Centre Rasi Che Neet Key 07-12-23Deena chemistNo ratings yet
- DPT 31 Xii Centre Rasi Che Iit Key 07-12-23Document4 pagesDPT 31 Xii Centre Rasi Che Iit Key 07-12-23Deena chemistNo ratings yet
- LT DPT 15 Jee 21.02.2024 KeyDocument1 pageLT DPT 15 Jee 21.02.2024 KeyDeena chemistNo ratings yet
- Revision Schedule 23-24Document22 pagesRevision Schedule 23-24Deena chemistNo ratings yet
- DPT 31 Xii Centre Rasi Che Iit 07-12-23Document4 pagesDPT 31 Xii Centre Rasi Che Iit 07-12-23Deena chemistNo ratings yet
- Electrochemistry 45 KeyDocument10 pagesElectrochemistry 45 KeyDeena chemistNo ratings yet
- Rasi WPT Xi Che Iit Key 01-1-1-24Document2 pagesRasi WPT Xi Che Iit Key 01-1-1-24Deena chemistNo ratings yet
- Coordination WSDocument3 pagesCoordination WSDeena chemistNo ratings yet
- LT DPT Jee Key 22.02.24Document1 pageLT DPT Jee Key 22.02.24Deena chemistNo ratings yet
- LT RPT Jee Phy 18.02.24Document4 pagesLT RPT Jee Phy 18.02.24Deena chemistNo ratings yet
- LT RPT2 Jee Che 18-02-24Document2 pagesLT RPT2 Jee Che 18-02-24Deena chemistNo ratings yet
- WPT CRP Xi Che Neet Key 18-02-24Document6 pagesWPT CRP Xi Che Neet Key 18-02-24Deena chemistNo ratings yet
- Xi ND Phy Iit CPT 19.02.24Document4 pagesXi ND Phy Iit CPT 19.02.24Deena chemistNo ratings yet
- Xi ND CPT ZoologyDocument4 pagesXi ND CPT ZoologyDeena chemistNo ratings yet
- Xi Rasi Phy Iit WPT 19.02.24 KeyDocument1 pageXi Rasi Phy Iit WPT 19.02.24 KeyDeena chemistNo ratings yet
- LT Jee DPT 15.02.24Document3 pagesLT Jee DPT 15.02.24Deena chemistNo ratings yet
- X ND WPT Che 1 17-10-22Document1 pageX ND WPT Che 1 17-10-22Deena chemistNo ratings yet
- Jee GrandDocument16 pagesJee GrandDeena chemistNo ratings yet
- Dptchem & Zoo01.2024Document2 pagesDptchem & Zoo01.2024Deena chemistNo ratings yet
- WPT Xi Centre Che Neet Key 21-11-23Document4 pagesWPT Xi Centre Che Neet Key 21-11-23Deena chemistNo ratings yet
- F BlockDocument10 pagesF BlockDeena chemistNo ratings yet
- CPT Rasi Xi Che NeetDocument5 pagesCPT Rasi Xi Che NeetDeena chemistNo ratings yet
- Hose, Tools &: AccessoriesDocument12 pagesHose, Tools &: AccessoriesYing Kei ChanNo ratings yet
- Spacer FabricDocument31 pagesSpacer FabricEric Chambers100% (1)
- Super Lube High Temperature Extreme Pressure (EP) Grease With Syncolon (PTFE)Document1 pageSuper Lube High Temperature Extreme Pressure (EP) Grease With Syncolon (PTFE)mohawxz357No ratings yet
- Formulating With Associative Rheology ModifiersDocument6 pagesFormulating With Associative Rheology ModifiersCereliaNo ratings yet
- Piping Materials ChartDocument1 pagePiping Materials ChartAitazaz AhsanNo ratings yet
- Introduction To SiliconesDocument5 pagesIntroduction To SiliconesGary NicholsonNo ratings yet
- Brewing With Cannabis Sativa Vs Humulus Lupulus A ReviewDocument9 pagesBrewing With Cannabis Sativa Vs Humulus Lupulus A ReviewJusepe Hbk LópezNo ratings yet
- Role of Digestive EnzymesDocument8 pagesRole of Digestive EnzymesvasaviNo ratings yet
- Acrylic: Alur Proses Pembuatan Serat AcrylicDocument7 pagesAcrylic: Alur Proses Pembuatan Serat AcrylicFathimatuz ZahroNo ratings yet
- MDL-7202A (2) - UnlockedDocument38 pagesMDL-7202A (2) - UnlockedRohan KabirNo ratings yet
- ScanRope Marine KatalogDocument16 pagesScanRope Marine KatalogMasterPie1950No ratings yet
- ASTM A380-99e1 Ðâ ÖÁã Þ¡ Éè ºÍÏ Í ÄÇåÏ ºÍ Ý (Ó ÎÄ) PDFDocument12 pagesASTM A380-99e1 Ðâ ÖÁã Þ¡ Éè ºÍÏ Í ÄÇåÏ ºÍ Ý (Ó ÎÄ) PDFcvazquez999No ratings yet
- Project D - Food Packaging in BruneiDocument4 pagesProject D - Food Packaging in Bruneiapi-3858849No ratings yet
- VW2.8.1 en 2009-12-01Document17 pagesVW2.8.1 en 2009-12-01mehmet ustunNo ratings yet
- TTQC 2 Assignment PDFDocument25 pagesTTQC 2 Assignment PDFJatul Akmam RahiNo ratings yet
- Any Aircraft Showing The Locations of The Composite Part 2. Advantages and Disadvantages of Using A CompositeDocument13 pagesAny Aircraft Showing The Locations of The Composite Part 2. Advantages and Disadvantages of Using A CompositeQueen CryNo ratings yet
- Oxford University Press - Online Resource Centre - Multiple Choice QuestionsDocument5 pagesOxford University Press - Online Resource Centre - Multiple Choice QuestionsHUAWEI HUAWEINo ratings yet
- Overview of GRP Pipes PDFDocument10 pagesOverview of GRP Pipes PDFamlanfacebookNo ratings yet
- SDS 2080 Penguard MidcoatDocument18 pagesSDS 2080 Penguard Midcoatmwazizi.torrNo ratings yet
- Advanced Wound DressingsDocument32 pagesAdvanced Wound DressingskkmNo ratings yet
- Fabric Powerpoint 19Document55 pagesFabric Powerpoint 19Japhet GatchoNo ratings yet
- Garlock-Rubber Expansion-Joints - Installation and Maint Manual PDFDocument20 pagesGarlock-Rubber Expansion-Joints - Installation and Maint Manual PDFsammar_10No ratings yet
- Chapter 6 Introduction To Autonomic PharmacologyDocument30 pagesChapter 6 Introduction To Autonomic PharmacologyImrana AamirNo ratings yet
- Schon Product List PDF 1Document14 pagesSchon Product List PDF 1vishal vishalNo ratings yet
- ACRYLONITRIELDocument86 pagesACRYLONITRIELPuja Banchu100% (1)
- Intraocular Lens Glistenings PDFDocument2 pagesIntraocular Lens Glistenings PDFAndreea L. MihalceaNo ratings yet
- Plasticizer SDocument28 pagesPlasticizer SM Arslan AshrafNo ratings yet
- Banana Paper - WikipediaDocument6 pagesBanana Paper - WikipediaXhanto FiliaNo ratings yet
- V Belt ManualDocument116 pagesV Belt ManualPankaj Pandey100% (1)