Professional Documents
Culture Documents
Tacrolimus COA
Uploaded by
willyvh990 ratings0% found this document useful (0 votes)
19 views1 pageCERTIFICADO DE CALIDAD TACROLIMUS
Original Title
Tacrolimus-COA
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCERTIFICADO DE CALIDAD TACROLIMUS
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
19 views1 pageTacrolimus COA
Uploaded by
willyvh99CERTIFICADO DE CALIDAD TACROLIMUS
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
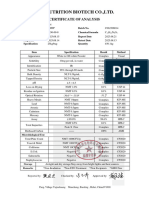
NORTH CHINA PHARMACEUTICAL HUASHENG CO., LTD.
CERTIFICATE OF ANALYSIS
Add: No. 8 Yangzi Road, Shijiazhuang Economic & Technological Development Zone, Hebei 052160, China
Tel:+86-311-83090290 Fax:+86-311-83090280 Website: http://www.ncpchs.com E-mail:mail@ncpchs.com
Product name :Tacrolimus
Tacrolimus
Batch No. : HNTN2301502U Manuf. date : 2023.01.19
Cert. No. : P-NT-01 Report date : 2023.02.10
Batch size : 11.478kg Retest date : 2026.01.18
Tests Specifications Results
Characters White crystals or white crystalline powder White crystalline powder
Identification A Infrared absorption Complies
B The retention time of the major peak of
the Sample solution corresponds to that of
the Standard solution as obtained in the Complies
Assay
Residue on ignition NMT 0.1% 0.05%
Optical rotation -110°to -115° -111°
Water NMT 4.0% 2.1%
Organic impurities
Ascomycin 19-epimer NMT 0.1% Not detected
Ascomycin NMT 0.50% Not detected
Desmethyl tacrolimus NMT 0.1% Not detected
Tacrolimus 8-epimer NMT 0.15% Not detected
Tacrolimus 8-propyl analog NMT 0.15% Not detected
Any individual unspecified NMT 0.1%
impurity Not detected
Total impurities NMT 1.0% Not detected
Assay(on the anhydrous and 98.0~102.0%
solvent-free basis) 100.1%
Residual solvent
Ethanol NMT 0.5% Not detected
Acetone NMT 0.5% Not detected
Ethyl acetate NMT 0.5% 0.003%
Isopropyl ether NMT 0.05% Not detected
Co n cl u si o n : Co mp l i es wit h cu r r en t USP
Reporter: Manager of QC division: Qualified Person:
You might also like
- COA Elacestrant Dihydrochloride Shandongkehui - 20240120221842Document2 pagesCOA Elacestrant Dihydrochloride Shandongkehui - 20240120221842rashidulhasan789No ratings yet
- Cholecalciferol RM COA 05Document1 pageCholecalciferol RM COA 05ASHOK KUMAR LENKA100% (1)
- COA of TirzepatideDocument1 pageCOA of TirzepatideskytechbridgeNo ratings yet
- PrintDocument2 pagesPrintShorup GhoshNo ratings yet
- Porous Prilled Ammonium Nitrate (PPAN) Certificate of AnalysisDocument1 pagePorous Prilled Ammonium Nitrate (PPAN) Certificate of AnalysisTan YoongNo ratings yet
- All Rac α Tocopheryl Acetate (Vitamin E Acetate) RM COA - 013Document2 pagesAll Rac α Tocopheryl Acetate (Vitamin E Acetate) RM COA - 013ASHOK KUMAR LENKANo ratings yet
- Certificate of Analysis Triclabendazole: Name of The ProductDocument2 pagesCertificate of Analysis Triclabendazole: Name of The Productbharath kumarNo ratings yet
- Celecoxib USP D90 Less Than 10microns Ex Aarti DrugsDocument1 pageCelecoxib USP D90 Less Than 10microns Ex Aarti Drugssuriana limNo ratings yet
- Certificate of Analysis: Quality ControlDocument2 pagesCertificate of Analysis: Quality ControlASHOK KUMAR LENKANo ratings yet
- Irvingia Gabonensis Seed Extract 10-1 (IGSE-220719)Document1 pageIrvingia Gabonensis Seed Extract 10-1 (IGSE-220719)Sophia XieNo ratings yet
- Certificate of Analysis Green CofeeDocument1 pageCertificate of Analysis Green CofeeEssamNo ratings yet
- Certificate of Analysis Green CofeeDocument1 pageCertificate of Analysis Green CofeeEssamNo ratings yet
- BQC kb03004 - Lowry Protein Assay Kit - ManualDocument16 pagesBQC kb03004 - Lowry Protein Assay Kit - ManualFaza FathiNo ratings yet
- Xi'an Haoxuan Bio-Tech Co.,Ltd: Certificate of AnylasisDocument1 pageXi'an Haoxuan Bio-Tech Co.,Ltd: Certificate of AnylasisNilsNo ratings yet
- 5-HTP CoaDocument1 page5-HTP Coawillyvh99No ratings yet
- 08 Coa Apl-Clp-01285-I-23Document1 page08 Coa Apl-Clp-01285-I-23bpharmbaNo ratings yet
- AKBA - 10% CoaDocument1 pageAKBA - 10% CoaDeepak VarmaNo ratings yet
- Rose Otto - RK0100Document11 pagesRose Otto - RK0100rocksanabNo ratings yet
- L (+) ORNITINA A3450 - 6d015448Document1 pageL (+) ORNITINA A3450 - 6d015448junio16No ratings yet
- Stability Data For Amitraz, 98%Document8 pagesStability Data For Amitraz, 98%Ronald NyamurowaNo ratings yet
- Irvingia Gabonensis Seed Extract 10-1 (IGSE-201209)Document1 pageIrvingia Gabonensis Seed Extract 10-1 (IGSE-201209)Sophia XieNo ratings yet
- Product Specification of Atracurium Besylate-USP43 Ex Lianyugang GuikeDocument2 pagesProduct Specification of Atracurium Besylate-USP43 Ex Lianyugang GuikeIhin SolihinNo ratings yet
- Oxyclozanide Vet BP 85: Certificate of AnalysisDocument168 pagesOxyclozanide Vet BP 85: Certificate of Analysisbharath kumarNo ratings yet
- 11 - Chapter 2 Analytical Method Validation AssayDocument44 pages11 - Chapter 2 Analytical Method Validation AssayLaura GuarguatiNo ratings yet
- COA Prilocaine HCL DST 2Document1 pageCOA Prilocaine HCL DST 2charlie ponteNo ratings yet
- Formato Certificado de AnálisisDocument2 pagesFormato Certificado de AnálisisMaria TejedaNo ratings yet
- Coa (VD3 Crystal Pharma Grade) - 2Document1 pageCoa (VD3 Crystal Pharma Grade) - 2Aleena RafeeqNo ratings yet
- CoA Sucralose - Supplier Tokped (Shandong Kanbo)Document1 pageCoA Sucralose - Supplier Tokped (Shandong Kanbo)Tantriyani GunadyNo ratings yet
- Certificate of Analysis: 17α-Hydroxy Progesterone AcetateDocument2 pagesCertificate of Analysis: 17α-Hydroxy Progesterone Acetatewindli2012No ratings yet
- Sorbitol 70% Non Crystallizing Liquid RM COADocument2 pagesSorbitol 70% Non Crystallizing Liquid RM COAASHOK KUMAR LENKANo ratings yet
- Coenzymeq10 SertDocument1 pageCoenzymeq10 SertAdward GloriousNo ratings yet
- Ca Pma 95Document1 pageCa Pma 95trungtamNo ratings yet
- Irvingia Gabonensis Seed Extract 10-1 IGSE-181011Document1 pageIrvingia Gabonensis Seed Extract 10-1 IGSE-181011Sophia XieNo ratings yet
- Chemo India Formulations Pvt. LTDDocument2 pagesChemo India Formulations Pvt. LTDjammuvenkatNo ratings yet
- Stability Data For Amitraz, 98%Document6 pagesStability Data For Amitraz, 98%Ronald NyamurowaNo ratings yet
- Lampiran 3 Dan 4 Proposal DisertasiDocument2 pagesLampiran 3 Dan 4 Proposal DisertasioktariyanaNo ratings yet
- Dossier - SS-Nicotinamide MononucleotideDocument16 pagesDossier - SS-Nicotinamide Mononucleotiderajha vikneshNo ratings yet
- Auro Labs LTD - Metf HCL USPDocument1 pageAuro Labs LTD - Metf HCL USPsuriana limNo ratings yet
- COA-Carnitine HCL-HengtaiDocument1 pageCOA-Carnitine HCL-Hengtaichurch.hrgNo ratings yet
- Layer4 Comfort - Chemical Breakthrough TimesDocument1 pageLayer4 Comfort - Chemical Breakthrough Timesbiomedicalsystems2022No ratings yet
- Antralina CoaDocument1 pageAntralina Coawillyvh99No ratings yet
- Vitamin A RM COADocument2 pagesVitamin A RM COAASHOK KUMAR LENKANo ratings yet
- Rankem SolventsDocument6 pagesRankem SolventsAmmar MohsinNo ratings yet
- GUID - 4 en-USDocument1 pageGUID - 4 en-USDilawar BakhtNo ratings yet
- Annex-C (Raw Material Specs)Document2 pagesAnnex-C (Raw Material Specs)Waqar AhmadNo ratings yet
- Soya Lecithim PowderDocument1 pageSoya Lecithim PowderASHOK KUMAR LENKANo ratings yet
- OOS 053 - 16.docx 01Document3 pagesOOS 053 - 16.docx 01ketone acidNo ratings yet
- SOL CBD Vape OilDocument2 pagesSOL CBD Vape OilHalcyonPublishingNo ratings yet
- GC CheckListsDocument3 pagesGC CheckListstuanlq73No ratings yet
- Thiamine Hydrochloride COA - 07Document2 pagesThiamine Hydrochloride COA - 07ASHOK KUMAR LENKANo ratings yet
- USP-NF Ethyl OleateDocument3 pagesUSP-NF Ethyl OleateMohamed BoumahrazNo ratings yet
- Certificate of Analysis: Sunflower Oil RefinedDocument1 pageCertificate of Analysis: Sunflower Oil RefinedBetrand MuNo ratings yet
- CoA of EgcgDocument1 pageCoA of EgcgMirna Candra RNo ratings yet
- Ascorbic Acid (Vitamin C)Document2 pagesAscorbic Acid (Vitamin C)ASHOK KUMAR LENKANo ratings yet
- Weizo DSRDocument1 pageWeizo DSRAshish SharmaNo ratings yet
- Ketamine HCL COA With MLT. SUPRIYADocument3 pagesKetamine HCL COA With MLT. SUPRIYARao Fahim NazarNo ratings yet
- Certificate of Analysis: Product: Other Name(s) : CAS No.: Code: Mol. Formula: Mol. WeightDocument1 pageCertificate of Analysis: Product: Other Name(s) : CAS No.: Code: Mol. Formula: Mol. WeightAnonymous yr4a85No ratings yet
- OGD Model QOS IR Tablet PDFDocument25 pagesOGD Model QOS IR Tablet PDFJoe Luis Villa MedinaNo ratings yet
- Pseudo BP RS OOS-017Document6 pagesPseudo BP RS OOS-017ketone acidNo ratings yet
- Compound Forming Extractants, Solvating Solvents and Inert Solvents: Iupac Chemical Data SeriesFrom EverandCompound Forming Extractants, Solvating Solvents and Inert Solvents: Iupac Chemical Data SeriesNo ratings yet
- KJB Answersheet Dpa-7 Goc Class 11Document3 pagesKJB Answersheet Dpa-7 Goc Class 11Gaurav KuntalNo ratings yet
- Carbenes ReactionsDocument10 pagesCarbenes Reactionsdeepakkr080No ratings yet
- Fundamentals of CombustionDocument16 pagesFundamentals of CombustionSeindahNyaNo ratings yet
- Castor Oil A Vital Industrial Raw MaterialDocument6 pagesCastor Oil A Vital Industrial Raw Materialonemahmud100% (2)
- Basic ShampooDocument9 pagesBasic Shampoosukriti biswas100% (1)
- Intrastat Receipts Dec 2016Document27 pagesIntrastat Receipts Dec 2016darapuNo ratings yet
- Chemsheets GCSE 1231 AlkanesDocument2 pagesChemsheets GCSE 1231 AlkanesRobinNo ratings yet
- Chemsheets GCSE 1105 (Titrations 1) ANS 93ghsDocument2 pagesChemsheets GCSE 1105 (Titrations 1) ANS 93ghs71700% (1)
- Making Salts: Neutralisation ReactionsDocument4 pagesMaking Salts: Neutralisation ReactionsPedro Moreno de SouzaNo ratings yet
- Catalytic Reduction With HydrazineDocument3 pagesCatalytic Reduction With HydrazineOgnian DimitrovNo ratings yet
- Chelated MineralsDocument24 pagesChelated MineralsGeorge DominicNo ratings yet
- Chem - Report 1Document7 pagesChem - Report 1Udaya ZorroNo ratings yet
- Chelate Effect (Recovered)Document13 pagesChelate Effect (Recovered)Ishu AttriNo ratings yet
- Solutions of Soaps in Organic SolventsDocument4 pagesSolutions of Soaps in Organic SolventsSandry KesumaNo ratings yet
- Chem ch10Document16 pagesChem ch10ChandlerNo ratings yet
- Colorimetric Determination PH PDFDocument8 pagesColorimetric Determination PH PDFscsa31619No ratings yet
- Other Phosphate Fertilizers - Part 1: Enriched SuperphosphateDocument10 pagesOther Phosphate Fertilizers - Part 1: Enriched SuperphosphatetegararazaqNo ratings yet
- Ionic and Covalent BondsDocument9 pagesIonic and Covalent BondsJad SuleimanNo ratings yet
- Class 12 Chapter 13 AminesDocument48 pagesClass 12 Chapter 13 Amineskumariakanksha276No ratings yet
- Presentation 9Document8 pagesPresentation 9sun shineNo ratings yet
- Tiamina, IPDocument4 pagesTiamina, IPmagicianchemistNo ratings yet
- Alkalinity Relationships in Water ChemistryDocument3 pagesAlkalinity Relationships in Water ChemistrytinuvalsapaulNo ratings yet
- LGCS Pharma Catalogue 2013 2014Document277 pagesLGCS Pharma Catalogue 2013 2014ramaiaNo ratings yet
- Carbohydrates NotesDocument9 pagesCarbohydrates NotesAshley Saron100% (1)
- Separation and Identification of Amino Acids by Paper ChromatographyDocument4 pagesSeparation and Identification of Amino Acids by Paper Chromatographyroxannediana86% (14)
- A Textbook of Organic Chemistry 1923Document935 pagesA Textbook of Organic Chemistry 1923Basharat RasheedNo ratings yet
- VJC H2 Chem P3 AnsDocument22 pagesVJC H2 Chem P3 Ansclarissa yeoNo ratings yet
- This Study Resource Was: Bgmea University of Fashion and TechnologyDocument9 pagesThis Study Resource Was: Bgmea University of Fashion and TechnologyRj SajolNo ratings yet
- Binder 2 EdexcelDocument9 pagesBinder 2 EdexcelahmedNo ratings yet