Professional Documents
Culture Documents
HMIS 033b - HEALTH UNIT WEEKLY EPIDEMIOLOGICAL New Edit 28.8.2019
Uploaded by
OdetteOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
HMIS 033b - HEALTH UNIT WEEKLY EPIDEMIOLOGICAL New Edit 28.8.2019
Uploaded by
OdetteCopyright:
Available Formats
HMIS 033b:
HEALTH UNIT WEEKLY EPIDEMIOLOGICAL
SURVEILLANCE REPORT
DESCRIPTION AND INSTRUCTIONS
Objective: To report cases of notifiable diseases, other diseases of public health importance,
OPD attendances, eMTCT appointments, Malaria tested and treated, TB tested and
treated and stock balances of select tracer items.
Frequency: Every Monday of the Week in a calendar year
Copies: Three Copies (Triplicate). Triplicate stays at the health unit, duplicate is sent to the
Health-Sub-District Headquarters, the Original copy is sent to the District health office.
Responsibility: Health Unit In-charge
PROCEDURE:
1. All health units must report this information (Government, Private Health Providers and PNFP) to the
HSD and DHO. In addition, the health facility should report any other priority disease and/or events
as required by the District Health Officer.
2. The report should be clearly labeled to show the period covered i.e. date for the first (Monday) and
last day (Sunday) of the week for which the report is being made.
3. For each disease category indicate the number of new cases during the week (cases this week), the
number of deaths that occurred during the week (deaths this week) and those tested (tested cases),
tested positive (Pos(+ve) cases)
4. For Maternal deaths, Perinatal deaths, OPD and eMTCT summary, summary of malaria cases tested
and treated, summary of tuberculosis cases tested and treated during the week, tracer medicines -
stock balance, HIV testing kits, TB, & eMTCT drugs - stock balance, summary of Genexpert report
and summary of IPT initiation, refer to the SOPs for the case definitions and how to extract the data.
5. For Other conditions (EPC.), refer to the given table below of other priority diseases/conditions for the
codes when reporting using mTrac.
6. Refer to the SOPs for the case definitions and how to extract the data.
7. The health unit continues to report every week throughout the year whether there are cases or not
and this should take care of “zero” report.
8. Transcribe the data every week into HMIS form 033c (Health Unit Weekly Epidemiological Surveil-
lance Summary for the year) for the respective weeks. For example 10 cases with 2 deaths are re-
corded as 10 (2).
Print Version September 2019
HMIS 033b:
HEALTH UNIT WEEKLY EPIDEMIOLOGICAL
SURVEILLANCE REPORT
Health Facility Name.........................................................Week....... Month .......... Calendar Year: ............
SELECTED OTHER PRIORITY DISEASES UNDER IDSR (INTEGRATED DISEASE SURVEILLANCE AND RESPONSES)
Diseases/conditions
Epidemic Prone Diseases/ Disease of public health
Code targeted for elimina- Code Code
Conditions importance
tion or eradication
Chikungunya CG. Dracunculiasis DC. Diarrhoea with dehydration <5 DD.
Dengue DG. Onchocerciasis OC. Severe pneumonia <5 PN.
illness IL. Buruli ulcer BU. Human African Trypanosomiasis TX.
Lymphatic Filariasis LF. Trachoma TR.
Acute viral hepatitis HP.
Noma NO. Schistosomiasis SC.
Human due to
HN. Diphtheria DP.
a new subtype
Severe Acute Respiratory
SS. Pertussis (Whooping cough) WC.
Syndrome (SARS)
Brucellosis
Smallpox SP. BC.
Kala azar KA.
Nodding Syndrome NS.
Adverse Drug Reactions (ADR) AR.
Note: 1. The health unit continues to report every week throughout the year whether there are
cases or not and this should take care of “zero report”.
2. For standard case of all priority IDSR diseases and conditions refer to the
standard case operating procedures.
Print Version September 2019
HMIS 033b:
HEALTH UNIT WEEKLY EPIDEMIOLOGICAL
SURVEILLANCE REPORT
Date of Report ________ Period: From (Date) ______________ To (Date) __________________
Week No. (#) ________ Health Facility ___________________ Code ____ Parish ___________
Sub-county ______________________ HSD ______________ District ___________________
CASES AND DEATHS THIS WEEK
2.
2. DEATH.
Total Cases Tested Pos(+ve)
Code Total Death
1. CASES. this week Cases cases
this week
1 Malaria (diagnosed) MA.
2 Dysentery DY.
3 Severe Acute Respiratory Infection SA.
(SARI)
4 Acute Flaccid Paralysis AF.
5 Adverse Events Following Immuniza- AE.
tion (AEFI)
6 Animal Bites (suspected rabies) AB.
7 Bacterial Meningitis MG.
8 Cholera CH.
9 Guinea Worm GW.
10 Measles ME.
11 Neonatal tetanus NT.
12 Plague PL.
13 Typhoid Fever TF.
14 Hepatitis B HB.
15 Rifampicin resistant TB cases DR.
16 Yellow Fever YF.
17 Other Viral Hemorrhagic Fevers (EVD, VF.
MVD, RVF, CCHF)
18 Leprosy LP.
19 Anthrax AX.
20 Maternal death MD.
21 Macerated Still births MB.
22 Fresh Still Birth FB.
23 Early Neonatal deaths 0-7 days ND.
Print Version September 2019
HMIS 033b:
HEALTH UNIT WEEKLY EPIDEMIOLOGICAL
SURVEILLANCE REPORT
3. Other conditions (refer to the table below for the other priority diseases/conditions)
DEATH (EPD.) Pos(+ve)
CASES (EPC.) Code Total Cases this week Tested Cases
cases
Total Death this week
1
2
3
4
5
6
7
8
9
10
4. OPD AND EMTCT SUMMARY
t
pp
na
nts
so
me
er
e
nc
th
e
int
nc
Mo
da
po
da
ten
ap
CT
ten
ed
At
MT
At
iss
D
de
w
OP
TM
Ne
cte
TC
al
D
pe
OP
eM
To
Ex
Tick
upon
Feedback
APT. . . .
Print Version September 2019
TB.
MAT.
.
Su
.
sp
Cl ec
te
ien d
ts m
Ca ala
sc se ria
re st (fe
en
.
es ve
ed te r)
d
.
fo RD wi
rT th
B TP RD
in os
itiv T
OP e
.
.
Ne
w D Ca
se
Ca
an se
dR st
es s
te
ela d
p wi
.
se Mi th
d cr Mi
.
Ne iag os cr
w no c op os
co
an p
d se
d
yP
os y
i
Re No
lap an
dr tt tive
s eg es Ca
e
REPORT
.
Ba ted
HMIS 033b:
cte tre T B
ist
er ca se
s
se
.
rio atm c ed RD st
log en ase TN re
ica t eg ate
d
ss
lly ati
HEALTH UNIT WEEKLY
tar ve
5. SUMMARY OF MALARIA CASES TESTED AND TREATED
TB
.
Ca
.
wi ted RD
th ca se
G en s es
on TP
os
sT
re
ex re itiv ate
EPIDEMIOLOGICAL SURVEILLANCE
pe gis eC d
rt as
.
Nu ter Mi es
ed cr
mb os Tr
er rm co
py e ate
of ed Ne d
TB T B ga
ca tiv
co se
Mi
cr e Ca
nta os se
c
sT co sT
ts es py re
ted Po ate
tra
ce s itiv d
da eC
nd as
es
sc Tr
ree ea
ted
ne
6. SUMMARY OF TUBERCULOSIS CASES TESTED AND TREATED DURING THE WEEK
Tick
upon
Tick
upon
Feedback
Feedback
Print Version September 2019
ARV.
TRA.
.
De
ter
.
mi AC
ne
HIV
T(
AR 1& Ta
b
.
Vs 2s let
(Fi cre s)
xe enin
.
d– OR
DC gt S(
es
Lo eM t Sa
.
p T CT ck
of ina )
12 vir ets
0c /R
ap )
su itona Me
.
Ne les vir as
pe
.
Ml virap lle
les
/l(1 ine ts- Va
00 Pa c
ml sus
) pe ck cin
ns Am eD
Ten ion ox
.
os
.
-bo ici
Pa ofov
7. TRACER MEDICINES - STOCK BALANCE
cks ir / ttle llin es
of Lam of
30 10 Dis
tab ivudi pe
s ne De rsi
b
RH /D p le
.
ZE olu
.
HMIS 033b:
Bli teg (DM ot m 25
ste rav PA edr 0m
ro ir ) in oxy gT
f2 jec p ab
8t
ab tab ogr let
RH let
s le est s
.
Bli ero
ste IV n
SURVEILLANCE REPORT
ea
.
rs art
of esu cet
28 na ate
8. HIV TESTING KITS, TB, & eMTCT Drugs - STOCK BALANCE
tab te
R(7 lets 60
.
tab 5) H(
5 mg
lets 0) Z
(15 Fa v ials
HEALTH UNIT WEEKLY EPIDEMIOLOGICAL
.
0) B ns
list ida
ers rT
.
INH of ab
300 28 let
mg
Bli RD s
ste
rs T(
INH of Ma
1 28 lar
tab 00m tab ia)
lets gb lets Tes
list t
ers s
of
10
Tick
Tick
upon
upon
Feedback
Feedback
Print Version September 2019
GP.
TPT.
No
.
of
Nu sa
.
mb mp
er les
of tes
Ad No
u lt A of
ted
.
Nu RT sa
mp
.
mb cli
er en les
of ts
10. SUMMARY OF TPT INITIATION
ch ini rej
ild tia ec
ren ted ted
Nu To
m a TP ta
.
eli nd T lM
.
gib ber o ad TB
le fc ole de
for hil sc te
TP dre en cte
T n ts d
Nu 0– on To
on be m 4y AR ta
.
TP r o rs Tw lN
T fc wh ho o.
hil Rif
HMIS 033b:
oa sta
dr
en re rte R
0– c on dT
4y tac PT No
rs ts .o
wh of fe
.
oa TB rro
re
SURVEILLANCE REPORT
co pa rs/
nta tie inv
cts nts alid
of res
TB ult
pa No
.G s
tie .
HEALTH UNIT WEEKLY EPIDEMIOLOGICAL
nts ene
ini Xp
tia ert
ted
9. SUMMARY OF GENEXPERT REPORT FOR GENEXPERT SITES ONLY
mo
du
les
No wo
of rki
n
car
trid g
ges
rem
ain
ing
Tick
upon
Tick
upon
Feedback
Feedback
Print Version September 2019
You might also like
- Substance Abuse Counseling Complete 5th EditionDocument379 pagesSubstance Abuse Counseling Complete 5th Editionnintendoagekid83% (6)
- 19 Calisthenics For The SaxophonistsDocument1 page19 Calisthenics For The Saxophonistsetdejazz67% (3)
- Ra 9003Document16 pagesRa 9003Calvin Jr. Wong100% (1)
- Pandemic Preparedness PlanDocument40 pagesPandemic Preparedness PlanMurali Vallipuranathan100% (2)
- Examination of The Shoulder The Complete Guide PDFDocument2 pagesExamination of The Shoulder The Complete Guide PDFJasmine0% (2)
- Best Practices in Operational Risks ManagementDocument44 pagesBest Practices in Operational Risks ManagementKETENo ratings yet
- DR - AC Ethical and Legal Issues in Midwifery Practice 2021Document91 pagesDR - AC Ethical and Legal Issues in Midwifery Practice 2021dr.anu RkNo ratings yet
- Operations & Maintenance Handbook - LPG Storage Facilities (Propane Research Council)Document176 pagesOperations & Maintenance Handbook - LPG Storage Facilities (Propane Research Council)KannanGKNo ratings yet
- DexamethasoneDocument4 pagesDexamethasoneMits Valencia Karlsson0% (2)
- Nonspeech Oral Motor Exercises: Theory and Evidence Against Their UseDocument11 pagesNonspeech Oral Motor Exercises: Theory and Evidence Against Their UseHeriberto RangelNo ratings yet
- Zero Reporting TemplateDocument1 pageZero Reporting Templatelance tabinas100% (2)
- 100 - So v. Tacla Jr.Document2 pages100 - So v. Tacla Jr.Alexis Elaine BeaNo ratings yet
- Immunopatologi Sepsis - DR Nur Farhanah SPPD K-PTIDocument29 pagesImmunopatologi Sepsis - DR Nur Farhanah SPPD K-PTIanita tri hastutiNo ratings yet
- Immunology Syllabus-2015 PDFDocument307 pagesImmunology Syllabus-2015 PDFLorena BravoNo ratings yet
- DM COVID Webinar 15 April 2020 Final - Prof Ketut SuastikaDocument38 pagesDM COVID Webinar 15 April 2020 Final - Prof Ketut SuastikaBudi RiyantoNo ratings yet
- Diagnosis and Management of COVID-19 Disease: Public HealthDocument4 pagesDiagnosis and Management of COVID-19 Disease: Public HealthSam HaileyNo ratings yet
- Diagnosis and Management of COVID-19 Disease: Public HealthDocument4 pagesDiagnosis and Management of COVID-19 Disease: Public HealthLOLOLONo ratings yet
- Covid 19Document53 pagesCovid 19Triangle Skeptics100% (2)
- Case Discussion: Tanaphon Kusonthomarat, 600710062 6th Year Medical Student, CMUDocument46 pagesCase Discussion: Tanaphon Kusonthomarat, 600710062 6th Year Medical Student, CMUICETNPNo ratings yet
- PHEM ReportDocument2 pagesPHEM ReportmekuriaNo ratings yet
- Data Visualization and Analyzation of COVID SynopsisDocument11 pagesData Visualization and Analyzation of COVID SynopsisDhananjaya dhanuNo ratings yet
- COVID 19 A Brief Clinical OverviewDocument5 pagesCOVID 19 A Brief Clinical OverviewgygyNo ratings yet
- Asita ElengoeDocument7 pagesAsita ElengoeSaodahSheikhHassanNo ratings yet
- 3 MICRO 12 - Emerging and Re-Emerging Infectious Diseases - Dr. RosarioDocument7 pages3 MICRO 12 - Emerging and Re-Emerging Infectious Diseases - Dr. RosarioyayayanizaNo ratings yet
- 2781 2783Document3 pages2781 2783Manuel Angel G BNo ratings yet
- AIDS: Clinical Manifestations: Gregory J Dore, David A CooperDocument8 pagesAIDS: Clinical Manifestations: Gregory J Dore, David A CooperFrancisco BecerraNo ratings yet
- Abstract: COVID-19, Also Known As Coronavirus Disease, Is A Respiratory InfectionDocument25 pagesAbstract: COVID-19, Also Known As Coronavirus Disease, Is A Respiratory Infectionamr amerNo ratings yet
- Nerologycal ComplicationsDocument2 pagesNerologycal Complicationspsicodiagnostico.pirovanoNo ratings yet
- Herpes Zoster in COVID19Document2 pagesHerpes Zoster in COVID19Vienesha Aress ShaNo ratings yet
- Covid-19 Malaysia Sars-Cov-2: KeywordsDocument3 pagesCovid-19 Malaysia Sars-Cov-2: KeywordsBellaNo ratings yet
- Endemic Diseases: The Supervisor: Fatima Hamaden Students Name: Manar Hamdan Batoul Hamad Amjad AbdullahDocument38 pagesEndemic Diseases: The Supervisor: Fatima Hamaden Students Name: Manar Hamdan Batoul Hamad Amjad AbdullahWek KivNo ratings yet
- Diagnostics of Acute Rheumatic Liquorad (Literature Review)Document4 pagesDiagnostics of Acute Rheumatic Liquorad (Literature Review)Central Asian StudiesNo ratings yet
- COVID-19 and Guillain-Barré Syndrome, A Single-Center Prospective Case Series With A 1-Year Follow-UpDocument8 pagesCOVID-19 and Guillain-Barré Syndrome, A Single-Center Prospective Case Series With A 1-Year Follow-UpLuciano RuizNo ratings yet
- CHN Definion JerickDocument6 pagesCHN Definion JerickRickNo ratings yet
- Molecular Review Sars CovDocument11 pagesMolecular Review Sars CovbudiNo ratings yet
- NCP - Risk For InfectionDocument3 pagesNCP - Risk For InfectionIRISH CACAYANNo ratings yet
- The Role of Emergency Radiology in COVID-19Document13 pagesThe Role of Emergency Radiology in COVID-19ViviApriliaNo ratings yet
- Clabsi DR - RonaldDocument19 pagesClabsi DR - RonaldsilviNo ratings yet
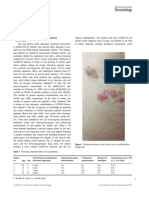
- Epidemiological Description of Chikungunya Virus Outbreak in Dire Dawa Administrative City, Eastern Ethiopia, 2019Document5 pagesEpidemiological Description of Chikungunya Virus Outbreak in Dire Dawa Administrative City, Eastern Ethiopia, 2019Nigatu AdmasuNo ratings yet
- Cutaneous Hyperaesthesia in SARS CoV-2 Infection Rare But Not Unique Clinical ManifestationDocument2 pagesCutaneous Hyperaesthesia in SARS CoV-2 Infection Rare But Not Unique Clinical ManifestationfikarNo ratings yet
- Disseminated Tuberculosis in An AIDS/HIV-Infected Patient: AbstractDocument3 pagesDisseminated Tuberculosis in An AIDS/HIV-Infected Patient: AbstractAmelia Fitria DewiNo ratings yet
- Comm Disease Assignment 5 Handout - INFECTIOUS-DISEASE-REPORTABLE-FLYERDocument6 pagesComm Disease Assignment 5 Handout - INFECTIOUS-DISEASE-REPORTABLE-FLYERUgochi 'Gucci' NdubuisiNo ratings yet
- Epidemic Investigation - Keshab RijalDocument8 pagesEpidemic Investigation - Keshab RijalKeshab RijalNo ratings yet
- Fulltext - Ams v5 Id1040Document4 pagesFulltext - Ams v5 Id1040yaneemayNo ratings yet
- Preprint Not Peer Reviewed: Spillover Effect of COVID19 On The Global Economy-A ReviewDocument16 pagesPreprint Not Peer Reviewed: Spillover Effect of COVID19 On The Global Economy-A ReviewMuskan GargNo ratings yet
- AACE Clinical Case Reports: Manthan Pandya, MD, Geethika Thota, MD, Xiangbing Wang, MD, Hongxiu Luo, MDDocument3 pagesAACE Clinical Case Reports: Manthan Pandya, MD, Geethika Thota, MD, Xiangbing Wang, MD, Hongxiu Luo, MDSameerNo ratings yet
- 6-Rheumatic Heart DiseaseDocument18 pages6-Rheumatic Heart DiseaseDr. Tonmoy BiswasNo ratings yet
- Covid 19 Guidelines April 2021Document42 pagesCovid 19 Guidelines April 2021K.sushmaNo ratings yet
- Two Emergent Diseases-Communicable DiseasesDocument1 pageTwo Emergent Diseases-Communicable DiseasesmariaalmiramsaritaNo ratings yet
- Dengue FeverDocument13 pagesDengue Feverjunathancortez123No ratings yet
- SLE NewDocument12 pagesSLE NewarifbudipraNo ratings yet
- The Beast 2020Document43 pagesThe Beast 2020Toma BichirNo ratings yet
- Ch. 16 - Disease and Epidemiology (Microbiology)Document46 pagesCh. 16 - Disease and Epidemiology (Microbiology)Gia Phong VuNo ratings yet
- Antibiotics 10 01189Document15 pagesAntibiotics 10 01189ApoloNo ratings yet
- SARS CoV 2, The Virus That Causes COVID 19 - Cytometry and The New Challenge For Global HealthDocument4 pagesSARS CoV 2, The Virus That Causes COVID 19 - Cytometry and The New Challenge For Global HealthSusanyi ErvinNo ratings yet
- Pertemuan 13 - Epidemiologi Penyakit New Emerging DiseaseDocument42 pagesPertemuan 13 - Epidemiologi Penyakit New Emerging DiseasePutti AnnisaNo ratings yet
- The Theory of Evolution Explained With The CoronavirusDocument6 pagesThe Theory of Evolution Explained With The CoronavirusdanielaNo ratings yet
- Factors of Severity in Patients With COVID-19: Cytokine/Chemokine Concentrations, Viral Load, and Antibody ResponsesDocument7 pagesFactors of Severity in Patients With COVID-19: Cytokine/Chemokine Concentrations, Viral Load, and Antibody ResponsesdariusNo ratings yet
- Covid 19 Review PaperDocument25 pagesCovid 19 Review PaperLeena MuniandyNo ratings yet
- Perioperative Management of Suspected/ Confirmed Cases of COVID-19Document13 pagesPerioperative Management of Suspected/ Confirmed Cases of COVID-19Dimensi FKUNHASNo ratings yet
- Perioperative Management of Suspected/ Confirmed Cases of COVID-19Document13 pagesPerioperative Management of Suspected/ Confirmed Cases of COVID-19fahadNo ratings yet
- Atow 422 01 PDFDocument13 pagesAtow 422 01 PDFfahadNo ratings yet
- Dengue Situation V2 - 0Document64 pagesDengue Situation V2 - 0Mitha Aulia IINo ratings yet
- VACCINESDocument20 pagesVACCINESSomesh MehraNo ratings yet
- DHIS Monthly Reporting Form PHC FacilitiesDocument4 pagesDHIS Monthly Reporting Form PHC FacilitiesVampire OnFireNo ratings yet
- PIIS0163445320302176Document2 pagesPIIS0163445320302176Hanan ArielNo ratings yet
- Immuno-Epidemiology and Pathophysiology of Coronavirus Disease 2019 (COVID-19)Document15 pagesImmuno-Epidemiology and Pathophysiology of Coronavirus Disease 2019 (COVID-19)KEEP CALM studiosNo ratings yet
- CPH Lab Activity 3 Group 4Document34 pagesCPH Lab Activity 3 Group 4Shane G.No ratings yet
- Dengue Fever: State-of-the-Art Symptoms and DiagnosisDocument6 pagesDengue Fever: State-of-the-Art Symptoms and Diagnosisajeet kumarNo ratings yet
- Perilaku Masyarak AT Terhadap Kejadian DBD Di Kecamata N Ciracas Jakarta TimurDocument13 pagesPerilaku Masyarak AT Terhadap Kejadian DBD Di Kecamata N Ciracas Jakarta TimurGde Wartama KepakisanNo ratings yet
- Neuroimaging of Covid-19. First Insights based on Clinical CasesFrom EverandNeuroimaging of Covid-19. First Insights based on Clinical CasesSimonetta Gerevini M.D.No ratings yet
- Purpose of The JobDocument3 pagesPurpose of The JobOdetteNo ratings yet
- The Little Cafe Menu DesignDocument4 pagesThe Little Cafe Menu DesignOdetteNo ratings yet
- HMIS 105 Health Unit Outpatient Monthly ReportDocument32 pagesHMIS 105 Health Unit Outpatient Monthly ReportOdetteNo ratings yet
- Pres 2 PastpapersDocument190 pagesPres 2 PastpapersOdetteNo ratings yet
- MLI QRTRDocument1 pageMLI QRTROdetteNo ratings yet
- MJAP Job Advert October 2022.-1Document7 pagesMJAP Job Advert October 2022.-1OdetteNo ratings yet
- Employment Opportunities With The Petroleum Authority of UgandaDocument31 pagesEmployment Opportunities With The Petroleum Authority of UgandaOdetteNo ratings yet
- M C H: Uganda: Aternal and Hild EalthDocument4 pagesM C H: Uganda: Aternal and Hild EalthOdetteNo ratings yet
- Obstetric DIC 2009 PDFDocument10 pagesObstetric DIC 2009 PDFOdetteNo ratings yet
- Blood Reviews: Jecko Thachil, Cheng-Hock TohDocument10 pagesBlood Reviews: Jecko Thachil, Cheng-Hock TohOdetteNo ratings yet
- NEPADocument21 pagesNEPARiddhi DhumalNo ratings yet
- Experiment2 Data PostLab-2 AnsDocument3 pagesExperiment2 Data PostLab-2 AnsSylvia and EdwardsNo ratings yet
- Lemone/Burke/Bauldoff/Gubrud, Medical-Surgical Nursing 5Th Edition Test BankDocument41 pagesLemone/Burke/Bauldoff/Gubrud, Medical-Surgical Nursing 5Th Edition Test Banknurse homeNo ratings yet
- Nutrition of Market CattleDocument4 pagesNutrition of Market Cattleapi-303043588No ratings yet
- Bluebook PDFDocument39 pagesBluebook PDFDumitrache MihaelaNo ratings yet
- PWA To Be Completed at The Point of Work: Assets Affairs O&M DepartmentDocument2 pagesPWA To Be Completed at The Point of Work: Assets Affairs O&M DepartmentaamedNo ratings yet
- MM 24Document32 pagesMM 24worksheetbookNo ratings yet
- PharmacologyDocument6 pagesPharmacologyZubair KhanNo ratings yet
- Institutional Review Board/Independent Ethics Committee (Irb/Iec)Document40 pagesInstitutional Review Board/Independent Ethics Committee (Irb/Iec)Swati chauhanNo ratings yet
- Promotion Safe Med ChildrensDocument64 pagesPromotion Safe Med ChildrensAbdul khodir jaelani100% (1)
- PulpotecDocument5 pagesPulpotecRuel MarmolejoNo ratings yet
- Guidelines For The Management of Postterm Pregnancy: Journal of Perinatal Medicine February 2010Document10 pagesGuidelines For The Management of Postterm Pregnancy: Journal of Perinatal Medicine February 2010Hafif Fitra Alief SultanaNo ratings yet
- Fiqh (Jurisprudence) : Noor-ul-Idha Wa Najatul ArwaahDocument6 pagesFiqh (Jurisprudence) : Noor-ul-Idha Wa Najatul ArwaahNoor-uz-Zamaan AcademyNo ratings yet
- IEEE Code of EthicsDocument13 pagesIEEE Code of EthicsMarium RanaNo ratings yet
- Lesson 1Document32 pagesLesson 1Miles BarrozoNo ratings yet
- Special Requirements of Electronic Medical Record Systems in Obstetrics and GynecologyDocument4 pagesSpecial Requirements of Electronic Medical Record Systems in Obstetrics and GynecologysayansambitNo ratings yet
- Sika Top-Seal 107Document5 pagesSika Top-Seal 107Gibbs PerNo ratings yet
- Grade5-Health-Ed - Catch-Up-Friday (Week 5)Document3 pagesGrade5-Health-Ed - Catch-Up-Friday (Week 5)Jam SnowNo ratings yet
- Flux 2331-Zx MsdsDocument8 pagesFlux 2331-Zx Msdsibnu Groho Herry sampurnoNo ratings yet