Professional Documents
Culture Documents
Sodium Has A Half-Life of 15 Hours. If There Are 800 G of Na Initially, How Long Will It Take For 750 G of Na To Decay Study
Uploaded by
alasjoyOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Sodium Has A Half-Life of 15 Hours. If There Are 800 G of Na Initially, How Long Will It Take For 750 G of Na To Decay Study
Uploaded by
alasjoyCopyright:
Available Formats
for Teachers for Schools for Working Scholars for College Credit
Plans Courses , Credit , Degrees , Schools , Log in Sign Up &
! Half-Life Questions And Answers
Support
Sodium has a half-life of 15 hours. If
there are 800 g of Na initially, how long
will it take for...
" like | # dislike
Question: Become a
Sodium has a half-life of 15 hours. If there are 800 g of Na initially, how long will it member and
take for 750 g of Na to decay?
unlock all
Study Answers
Half-Life:
Try it risk-free for 30
A radioactive substance is the one that spontaneously disintegrates. It consists of days
heavy unstable nuclei that spontaneously decay into lighter, more stable nuclei.
The decay occurs spontaneously.
Try it risk-free
The half-life of a substance is the time in which half of the substance decays. It
depends on the nature of the substance and is independent of other parameters
in the case of nuclear decay.
Note that half-life is used for other reactions as well in biology and chemistry,
where it denotes the time of disintegration of half of the molecules. %
Ask a question
$ Answer and Explanation:
Our experts can answer
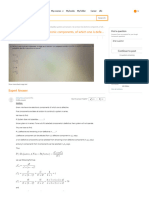
We are given: your tough homework and
study questions.
The half-life of Sodium, τ = 15 h
the initial mass of the sample, m0 = 800 g Ask a question
The mass decayed, m = 750 g
The mass of sodium remaining is:
m′ = m0 − m Search Answers
= 800 g − 750 g
= 50 g
What question do you need
&help with
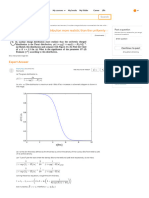
The amount of substance remaining after a time equal to n half-lives is given by
the equation:
m0
m′ =
2n
Here,
m0 is the initial mass of the substance.
m′ is the mass of the substance remaining after n half-lives.
After plugging the given values into the above equation, we have:
800 g
50 g =
2n
800 g
⇒ 2n =
50 g
⇒ 2n = 16
⇒ 2n = 24
⇒n= 4
Therefore, it will take ∆t = nτ = 4 × 15 h = 60 h for 750 g of sodium to decay, starting with an
initial mass of m0 = 800 g.
Learn more about this topic:
Half-life: Calculating Radioactive
Decay and Interpreting Decay
Graphs
from Chemistry 101: General Chemistry
Chapter 4 / Lesson 3
' 76K
Related to this Question
Related Answers Related Lessons Related Courses
The half-life of Calculate the How long would it 5 mg iodine(131)
radium is 1690 initial quality for take 312 g of the has a half life of
years. If 80 g is... the radioactive... sample to... 8.1 days. What...
Explore our homework questions and answers library
Search What question do you need help with? & Browse Browse by subject (
Plans About Us Support Download the
app
) * +
Student Solutions Blog Contact Support
Teacher Solutions Careers FAQ
Study.com for Get Your School Site Feedback
Schools Listed
Working Scholars
Working Scholars Teach For Us Bringing Tuition-Free
Solutions Press Center College to the
Community
© copyright 2003-2020 Study.com. All other trademarks and copyrights are the property of their respective owners. All rights reserved.
Terms of Use Privacy Policy DMCA Notice ADA Compliance Honor Code For Students
%
Ask a Homework Question -
Tutors available
You might also like
- Which Has The Larger Atomic Radius Phosphorus or Sulfur Explain.Document1 pageWhich Has The Larger Atomic Radius Phosphorus or Sulfur Explain.vanshgajiwalaNo ratings yet
- Silver Hypochlorite Formula, Solubility & Molar MassDocument1 pageSilver Hypochlorite Formula, Solubility & Molar MassJared Delos ReyesNo ratings yet
- Question: 3. The Radius of The Sun Is R 6.96x10 8 M, and The Distance BeDocument2 pagesQuestion: 3. The Radius of The Sun Is R 6.96x10 8 M, and The Distance BeMichael Sean BumanglagNo ratings yet
- Solved Hi Kinini, I Hope You Are Doing Well I Want Help in The FollowingDocument3 pagesSolved Hi Kinini, I Hope You Are Doing Well I Want Help in The FollowingMesso FrancisNo ratings yet
- Question: Q4. Bigbazar Has 1,000 Registered Gold Members Who Can ReDocument4 pagesQuestion: Q4. Bigbazar Has 1,000 Registered Gold Members Who Can ReAhammed UdoyNo ratings yet
- Find Study Resources: AnsweredDocument2 pagesFind Study Resources: AnsweredAnne GleindezNo ratings yet
- Question: An Aerobic Complete Mix-Reactor (No Recycle) With A Volume ofDocument2 pagesQuestion: An Aerobic Complete Mix-Reactor (No Recycle) With A Volume ofHmid AljbreNo ratings yet
- Old MathJax Webview Kindly Answer The Data Ang Com...Document10 pagesOld MathJax Webview Kindly Answer The Data Ang Com...LOREN MAE BULAYBULAYNo ratings yet
- JEE Physics Test Series 1 PDFDocument21 pagesJEE Physics Test Series 1 PDFAkil kumarNo ratings yet
- Apps Unit 1Document239 pagesApps Unit 1miskobukilic1100% (1)
- A Drum 6 In. in Diameter and 40 In. Long Contained...Document2 pagesA Drum 6 In. in Diameter and 40 In. Long Contained...panger moooNo ratings yet
- Seatwork 1. A Closed Surface Is Defined in Spheric...Document2 pagesSeatwork 1. A Closed Surface Is Defined in Spheric...Frederick DugayNo ratings yet
- Radioactivity HomeworkDocument8 pagesRadioactivity Homeworkfzg9w62y100% (1)
- For An Ideal Gas PV NRT Where N Is The Number O...Document3 pagesFor An Ideal Gas PV NRT Where N Is The Number O...Azina KhanNo ratings yet
- Determine The Maximum Allowable Torque That Can Be.Document3 pagesDetermine The Maximum Allowable Torque That Can Be.Din TescoNo ratings yet
- Step-By-Step ExplanationDocument3 pagesStep-By-Step ExplanationFrista Khaulanabila Adina PutriNo ratings yet
- Find Study Resources: AnsweredDocument2 pagesFind Study Resources: AnsweredAnne GleindezNo ratings yet
- Quesiton 6 AssigmentDocument6 pagesQuesiton 6 AssigmentMuhammad NaguibNo ratings yet
- 8.) An Explosive Liquid at Temperature 300 K Conta...Document2 pages8.) An Explosive Liquid at Temperature 300 K Conta...Azina Khan100% (1)
- Answers To Ut Homework Quest ChemistryDocument6 pagesAnswers To Ut Homework Quest Chemistryafetzhsse100% (1)
- Preliminary Work PDFDocument19 pagesPreliminary Work PDFcheng linNo ratings yet
- Sadler Maths Methods Unit 1Document288 pagesSadler Maths Methods Unit 1Edwin86% (7)
- AJ Sadler Methods Unit 1Document288 pagesAJ Sadler Methods Unit 1daniell.baii2526No ratings yet
- Mole Concept and Problems Solving Approaches in Life SciencesDocument21 pagesMole Concept and Problems Solving Approaches in Life SciencesHrithikNo ratings yet
- Solved - How Is The Initial Activity Rate of A Radioactive Subst...Document2 pagesSolved - How Is The Initial Activity Rate of A Radioactive Subst...Malik HammadNo ratings yet
- The Mean Activity Coefficients of HBR in Three Dil...Document2 pagesThe Mean Activity Coefficients of HBR in Three Dil...Robert Clark100% (1)
- Question: PA6. LO 2.2 Olivia's Apple Orchard Had The Following TransactioDocument2 pagesQuestion: PA6. LO 2.2 Olivia's Apple Orchard Had The Following TransactioMarie SamsonNo ratings yet
- Grade 7 Science Homework HelpDocument8 pagesGrade 7 Science Homework Helpafnoiaynmdfpew100% (1)
- 5090 Biology Example Candidate Responses Booklet 2014-1 PDFDocument120 pages5090 Biology Example Candidate Responses Booklet 2014-1 PDFEndeavor Med TutorsNo ratings yet
- Biology A Level No CourseworkDocument4 pagesBiology A Level No Courseworkafazapfjl100% (2)
- A Nuclear Charge Distribution More Realistic Than ...Document3 pagesA Nuclear Charge Distribution More Realistic Than ...Robert ClarkNo ratings yet
- L Iron Stand String Mechanical Vibrator Spring Bal...Document6 pagesL Iron Stand String Mechanical Vibrator Spring Bal...LOREN MAE BULAYBULAYNo ratings yet
- Modern Chemistry Homework 5-10 AnswersDocument7 pagesModern Chemistry Homework 5-10 Answersffutcfrmg100% (1)
- AJ Sadler Specialist Unit 3, 4Document371 pagesAJ Sadler Specialist Unit 3, 4bk3pgxrthp100% (1)
- 1 We Have Nine Electronic Components, of Which O Chegg ComDocument2 pages1 We Have Nine Electronic Components, of Which O Chegg ComMus'ab AlduqqahNo ratings yet
- Chemistry Homework Book AnswersDocument5 pagesChemistry Homework Book Answerssetyjokemyd3100% (1)
- Natural Radioactivity Homework AnswersDocument5 pagesNatural Radioactivity Homework Answersehrcyltif100% (1)
- Question: B. I. The Model For A Packed-Bed Reactor System Is Given As - 44Document1 pageQuestion: B. I. The Model For A Packed-Bed Reactor System Is Given As - 44Amirul IqmalNo ratings yet
- Specialist Unit 1, 2Document310 pagesSpecialist Unit 1, 2bk3pgxrthp100% (1)
- MoleconcepthighvaluenoteV1 2Document21 pagesMoleconcepthighvaluenoteV1 2khadeane wilsonNo ratings yet
- 3-11. If, Instead of The Observed Value of J - 1+...Document2 pages3-11. If, Instead of The Observed Value of J - 1+...Robert ClarkNo ratings yet
- Two Reservoirs Having Difference in Water Levels A...Document3 pagesTwo Reservoirs Having Difference in Water Levels A...Bishal Chandra PaulNo ratings yet
- The Average Admission Charge For A Movie Is $5.81. If The Distribution of Movie Admission Charges..Document1 pageThe Average Admission Charge For A Movie Is $5.81. If The Distribution of Movie Admission Charges..satish chandranNo ratings yet
- General Physics 2: San Fabian National High SchoolDocument10 pagesGeneral Physics 2: San Fabian National High SchoolAlexa ValdezNo ratings yet
- Determine The Memory Location Addressed by The Fol.Document2 pagesDetermine The Memory Location Addressed by The Fol.asddsa123No ratings yet
- A Safety Engineer Feels That 35% of All Industrial...Document2 pagesA Safety Engineer Feels That 35% of All Industrial...Hafiz Muhammad Umar AslamNo ratings yet
- Chem 30 Unit Plan - Cameron StuchlyDocument9 pagesChem 30 Unit Plan - Cameron Stuchlyapi-485518104No ratings yet
- 3-2. If The Surface of A Deformed Nucleus Is Given...Document2 pages3-2. If The Surface of A Deformed Nucleus Is Given...Robert ClarkNo ratings yet
- 16.8 Parte UnicaDocument20 pages16.8 Parte UnicaAbraao Zuza CostaNo ratings yet
- (Solved) at The Instant Shown, 60Document3 pages(Solved) at The Instant Shown, 60marcosbispolimaNo ratings yet
- Homework Helpers PhysicsDocument8 pagesHomework Helpers Physicsh66f9hzb100% (1)
- Com - Whatsapp.provider - Media Item 7c03ab33 A1d2 42a6 Ad9e 2a37a8bcbdb8Document5 pagesCom - Whatsapp.provider - Media Item 7c03ab33 A1d2 42a6 Ad9e 2a37a8bcbdb8Adedokun Opeyemi SodiqNo ratings yet
- A. Identify The Mode of Ring Closure For Each of T...Document3 pagesA. Identify The Mode of Ring Closure For Each of T...Robert ClarkNo ratings yet
- Chapter 8Document7 pagesChapter 8tere sariNo ratings yet
- Determine The Force in Members CD and DF of The Tr... !Document1 pageDetermine The Force in Members CD and DF of The Tr... !Erickson YanNo ratings yet
- Question: This VI Is Use To ND The Polynomial of The Transfer Functions deDocument3 pagesQuestion: This VI Is Use To ND The Polynomial of The Transfer Functions deDaryl Khent LorzanoNo ratings yet
- 1.4 IB Mindmap Template and Checklist SV PDFDocument3 pages1.4 IB Mindmap Template and Checklist SV PDFMario1984No ratings yet
- Conceptual Physics (PHYS 100) Fall 2008: TH THDocument22 pagesConceptual Physics (PHYS 100) Fall 2008: TH THSajadBishopNo ratings yet
- AQA Psychology BRILLIANT MODEL ANSWERS: Biopsychology AS and A-levelFrom EverandAQA Psychology BRILLIANT MODEL ANSWERS: Biopsychology AS and A-levelNo ratings yet
- E Stimation of HemoglobinDocument13 pagesE Stimation of HemoglobinSANANo ratings yet
- Non Heat Treatable Commercial-Purity Aluminium 1050 A: Chemical Composition Limits (In %) Aluminium 99,5% MinimumDocument1 pageNon Heat Treatable Commercial-Purity Aluminium 1050 A: Chemical Composition Limits (In %) Aluminium 99,5% Minimumprivate 2No ratings yet
- Solid State 48 70Document9 pagesSolid State 48 70Chetana PatilNo ratings yet
- SyllabusDocument38 pagesSyllabusहुडदंग हास्य कवि सम्मलेनNo ratings yet
- CA Lesson 2 AlkanesDocument31 pagesCA Lesson 2 AlkanesAlbaraaAliNo ratings yet
- BTech IT 2018-Onwards Jun-2021Document200 pagesBTech IT 2018-Onwards Jun-2021KittyPantherNo ratings yet
- Biology WorksheetDocument2 pagesBiology WorksheetAziyaNo ratings yet
- Charpy Test ReportDocument8 pagesCharpy Test ReportMuhammad ZulkarnainNo ratings yet
- Wastewater - Akash SahDocument24 pagesWastewater - Akash SahAkash Prasad100% (1)
- Mil PRF 7024Document10 pagesMil PRF 7024kidseismicNo ratings yet
- Consumer Guide CementDocument2 pagesConsumer Guide CementJojo Aboyme CorcillesNo ratings yet
- Welding EngineerDocument10 pagesWelding EngineerNnamdi Celestine NnamdiNo ratings yet
- JBL Setting Up An Aquarium enDocument40 pagesJBL Setting Up An Aquarium enRoy HarperNo ratings yet
- Date Tehnice - Regulator Gaz Filtru DN 15 - 65 - MadasDocument8 pagesDate Tehnice - Regulator Gaz Filtru DN 15 - 65 - Madasmishu35No ratings yet
- Thermal PollutionDocument15 pagesThermal PollutionArunniya SNo ratings yet
- Jomon - C14Document10 pagesJomon - C14Димитрије МарковићNo ratings yet
- Effect of Rice Husk Ash On Lime StabilizationDocument20 pagesEffect of Rice Husk Ash On Lime Stabilizationmyself_07101100% (1)
- Residential ComponentsDocument0 pagesResidential ComponentsChevronelleNo ratings yet
- Module in Forensic Chemistry Lesson 7Document26 pagesModule in Forensic Chemistry Lesson 7Anna Rose Valencia TallenoNo ratings yet
- Obtaining Pure Acetanilide From Crude Acetanilide by Recrystallization ProcessDocument5 pagesObtaining Pure Acetanilide From Crude Acetanilide by Recrystallization ProcessCharlot NavarroNo ratings yet
- Chapter 2 - A (Mechanical)Document142 pagesChapter 2 - A (Mechanical)osama shareetNo ratings yet
- Virtual Lab ElectrochemistryDocument5 pagesVirtual Lab ElectrochemistryRenneth Rea FloresNo ratings yet
- 2.5-V Integrated Reference Circuit: FeaturesDocument24 pages2.5-V Integrated Reference Circuit: FeaturesAnderson BorgesNo ratings yet
- BMP - (Class 01) - IntroductionDocument11 pagesBMP - (Class 01) - IntroductionAsesh PramanikNo ratings yet
- Specificatii Puffer IDMDocument48 pagesSpecificatii Puffer IDMIonut StavaracheNo ratings yet
- The Rice Wine Project: Presentation By: Jesbert Josh Osiana Grade 11 Aquamarine Biology - MsDocument6 pagesThe Rice Wine Project: Presentation By: Jesbert Josh Osiana Grade 11 Aquamarine Biology - MsShin KazueNo ratings yet
- IR Labor Law CapsuleDocument97 pagesIR Labor Law CapsuleVrajlal SapovadiaNo ratings yet
- NCERT Solutions For Class 7 Science Chapter 6Document4 pagesNCERT Solutions For Class 7 Science Chapter 6raju bhowalNo ratings yet
- Use of Amino Acids For Gold DissolutionDocument7 pagesUse of Amino Acids For Gold DissolutionCarlos PereaNo ratings yet
- Water Logging and Salinity ControlDocument13 pagesWater Logging and Salinity Controlpik MeNo ratings yet