Professional Documents
Culture Documents
Physical Sciences P2 Memo May 2022
Uploaded by
thabangaphanebusiness1Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Physical Sciences P2 Memo May 2022
Uploaded by
thabangaphanebusiness1Copyright:
Available Formats
NATIONAL SENIOR CERTIFICATE EXAMINATION
MAY 2022
PHYSICAL SCIENCES: PAPER II
MARKING GUIDELINES
Time: 3 hours 200 marks
These marking guidelines are prepared for use by examiners and sub-examiners,
all of whom are required to attend a standardisation meeting to ensure that the
guidelines are consistently interpreted and applied in the marking of candidates'
scripts.
The IEB will not enter into any discussions or correspondence about any marking
guidelines. It is acknowledged that there may be different views about some
matters of emphasis or detail in the guidelines. It is also recognised that, without
the benefit of attendance at a standardisation meeting, there may be different
interpretations of the application of the marking guidelines.
IEB Copyright © 2022 PLEASE TURN OVER
NATIONAL SENIOR CERTIFICATE: PHYSICAL SCIENCES: PAPER II – MARKING GUIDELINES – MAY Page 2 of 8
QUESTION 1 MULTIPLE CHOICE
1.1 B
1.2 D
1.3 C
1.4 B
1.5 A
1.6 B
1.7 D
1.8 D
1.9 B
1.10 C
QUESTION 2
2.1 4Na(s) + O2(g) → 2Na2O(s)
LHS
RHS
Balancing
States
2.2 • Ionic bonding
• involving the transfer of electrons (OR sodium loses electrons and oxygen
gains electrons)
2.3 • In order to melt sodium oxide, many (very) strong ionic bonds must be
broken
• This requires a large amount of energy
2.4 2.4.1 Na+ + e− → Na
2.4.2 • In a solid, the sodium and oxide ions are fixed in position in the
crystal lattice
• When molten, the ions are free to move
m
2.5 2.5.1 n (Na2O ) =
M
n (Na2O ) =
( 50 )
( 62)
n (Na2O) = 0,81 mol
2.5.2 The amount of solute per unit volume of solution.
( )
2.5.3 • n OH = n (NaOH) = ( 0,81)
− 2
1
= 1,62 mol
• c ( OH− ) =
n
=
(1,62 ) (sub) (conversion of V)
V ( 0,5 )
• ( )
c OH− = 3,24 mol·dm−3
(coe from Question 2.5.1)
2.5.4 Kw = H3O+ OH−
( )
10−14 = H3O+ ( 3,24 )
H3O = 3,09 10−15 mol·dm−3
+
(coe from Question 2.5.3)
IEB Copyright © 2022 PLEASE TURN OVER
NATIONAL SENIOR CERTIFICATE: PHYSICAL SCIENCES: PAPER II – MARKING GUIDELINES – MAY Page 3 of 8
QUESTION 3
27,6
3.1 3.1.1 Average rate = = 1,02 mg·min−1
33
3.1.2 • Bromine is the limiting reagent
• because there is rhodium left over after the reaction has come to
completion
3.1.3 10 min ( 2 min)
m
3.1.4 Average rate =
t
mi − (15 )
( 27,6 ) =
(10 )
mi = 291 mg
coe from 3.1.3
3.1.5
Lower gradient
Approximately one third change in mass
3.1.6 • A higher concentration in Experiment 1 means that there is a
greater number of particles per unit volume
• This causes a greater number of collisions per unit time
• Thus, there is a greater number of effective collisions per unit time
• and so Experiment 1 has a higher reaction rate
3.2 3.2.1 The net change in chemical potential energy of the system.
3.2.2 Negative
3.2.3 • The reaction rate will increase (uncontrollably)
• This might cause the water to boil OR it might cause the reaction
to finish too early OR be difficult to measure
IEB Copyright © 2022 PLEASE TURN OVER
NATIONAL SENIOR CERTIFICATE: PHYSICAL SCIENCES: PAPER II – MARKING GUIDELINES – MAY Page 4 of 8
QUESTION 4
C2H6 O2
2
4.1 4.1.1 Kc = (top) (bottom)
C2H4 H2O
2 2
C2H6 O2
2
4.1.2 Kc =
C2H4 H2O
2 2
C2H6 ( 0,08 )
2
( 0,025 ) =
( 0,4 ) ( 0,28 )
2 2
C2H6 = 0,0626 mol·dm−3
n ( C2H6 ) = cV = ( 0,0626 )(3 ) = 0,19 mol
4.1.3 The pressure was decreased (OR the volume was increased)
(only 1 mark for saying a change in pressure/volume)
4.1.4 When an external stress (change in pressure, temperature or
concentration) is applied to a system in dynamic chemical
equilibrium, the equilibrium point will change in such a way as to
counteract the stress.
All three bolded statements for two marks.
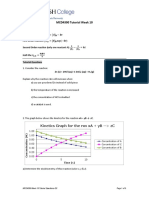
4.1.5 • Stress: increase in temperature
• Response: Le Châtelier's principle predicts the system will
counteract the stress and decrease the temperature
• Thus, the endothermic reaction is (initially) favoured as it
consumes heat
• From the graph, we can see that the concentration of reactants
(C2H4 + H2O) decreased and products (C2H6 + O2) increased
• Therefore, the forward reaction was favoured
• The forward reaction must be endothermic
4.1.6 No effect
4.2 4.2.1 No effect
4.2.2 pH is a measure of hydronium ion concentration at 25 °C.
4.2.3 • Stress: increase in concentration of Ca2+
• Le Châtelier's principle predicts the system will respond in order to
decrease the concentration of Ca2+
• Therefore, the reverse reaction is favoured as it consumes Ca2+
• The concentration of OH− decreases OR the concentration of H3O+
increases
• Therefore, the pH decreases
IEB Copyright © 2022 PLEASE TURN OVER
NATIONAL SENIOR CERTIFICATE: PHYSICAL SCIENCES: PAPER II – MARKING GUIDELINES – MAY Page 5 of 8
QUESTION 5
5.1 Any TWO of the following:
• Morgan used tap water instead of distilled water
• Morgan used a beaker instead of a volumetric flask
• Morgan added the HCN after the water was filled to the mark instead
of before
5.2 An acid that only ionises partially in an aqueous solution.
5.3 The acid can only donate one proton.
5.4 The reaction of a molecular substance with water to produce ions.
5.5 HCN + H2O ⇌ CN− + H3O+ (RHS)
5.6 Concentrations:
Reaction/Ratio HCN + H2O ⇌ CN− + H3O+
Initial conc 0,05 0 0
Change in conc –x +x +x
Equilibrium conc 0,05 – x x x
(ignore any value in the H2O column)
CN− H3O +
Ka =
HCN
( )( )
( 6,2 10 ) = (0,05 ( 6,2 10 ) = ((0,05
)( )
−10 x x −10 x x
OR
− x) )
H3O+ = x = 5,57 10−6 mol·dm−3
5.7 5.7.1 • na = caVa = ( 0,05)( 0,025) (sub) = 0,00125 mol
• nb = na = 0,00125 mol
nb ( 0,00125 )
• cb = = = 0,04 mol·dm−3
Vb ( 0,03344 )
5.7.2 Yellow
5.7.3 Phenolphthalein
5.7.4 A substance in which the hydrogen of an acid has been replaced by a
cation.
5.7.5 CN− + H2O ⇌ HCN + OH−
LHS
RHS
Reversible arrow
IEB Copyright © 2022 PLEASE TURN OVER
NATIONAL SENIOR CERTIFICATE: PHYSICAL SCIENCES: PAPER II – MARKING GUIDELINES – MAY Page 6 of 8
QUESTION 6
6.1 To provide a solid surface for electron transfer/loss
6.2 The gain of electrons.
6.3 2Cℓ− → Cℓ2 + 2e−
6.4 2Au3+ + 6Cℓ− → 2Au + 3Cℓ2
LHS
RHS
Balancing
6.5 6.5.1 • Completes the circuit
• Maintains electrical neutrality
6.5.2 (a) Decreases
(b) No effect
6.6 No
The graph is not a straight line (through the origin)
6.7 0
Ecell = Ecathode
0
− Eanode
0
0
Ecell = ( +1,42) − ( +1,36 )
0
E cell = 0,06 V
QUESTION 7
7.1 Electrical (potential) energy to chemical (potential) energy
7.2 Q
7.3 2Br− → Br2 + 2e−
7.4 7.4.1 The electrode at which reduction occurs.
7.4.2 A substance that accepts electrons.
7.4.3 Cu
7.4.4 • Cu2+ is a stronger oxidising agent than H2O (OR H2O is a weaker
oxidising agent than Cu2+)
• Therefore, Cu2+ is more likely to be reduced (OR H2O is less likely
to be reduced OR the rate of the Cu2+ reduction is much higher OR
the rate of the H2O reduction is much lower)
7.4.5 C
IEB Copyright © 2022 PLEASE TURN OVER
NATIONAL SENIOR CERTIFICATE: PHYSICAL SCIENCES: PAPER II – MARKING GUIDELINES – MAY Page 7 of 8
QUESTION 8
8.1 Haloalkanes (OR alkyl halides)
8.2 2−chlorobutane
2−chloro CH3
but H C H
ane
H C H
8.3 CH
H 3H H
H
H C H
C C C Br
H C
H H H
H H H C H
H C C C C
H Br
C H
H H H CH3longestcarbon chain: butane
H C H
2 ethyl groups on C2
H C H
Br on C1
H C H
H C H
CH
H 3H H
8.4 1−bromodiethylbutane
H C C C C (OR Br 1−bromo−2,2−diethylbutane)
1−bromo H H H
diethyl (OR 2,2−diethyl)
H C H
but
ane H C H
CH
8.5 They have the same3 molecular formula but different structural formulas (OR
different structures).
8.6 Chain
8.7 • Both compounds B and C have London forces (only)
• Compound C has a larger interacting surface
• leading to larger induced dipoles
• and thus stronger London forces (IMFs)
• More energy is required to overcome the intermolecular forces (OR
separate the particles) in compound C
• Thus, compound C has a higher boiling point
8.8 8.8.1 Substitution
8.8.2 (C2H5)3CCH2Br + NaOH → (C2H5)3CCH2OH + NaBr
NaBr
(C2H5)3CCH2OH
8.9 8.9.1 Dehydrohalogenation (OR dehydrobromination)
8.9.2 Double carbon-carbon bond
IEB Copyright © 2022 PLEASE TURN OVER
NATIONAL SENIOR CERTIFICATE: PHYSICAL SCIENCES: PAPER II – MARKING GUIDELINES – MAY Page 8 of 8
QUESTION 9
9.1 H2SO4
9.2 Propanoic acid
9.3 Alkenes
9.4
butyl prop functional group
9.5 9.5.1 • Combustion reactions are highly exothermic OR they release large
amounts of energy
• for a small amount of fuel (OR which is used for heat, light, or to
do some sort of work by the engine OR they are clean fuels)
9.5.2 C4H10O + 6O2 → 4CO2 + 5H2O
C4H10O
O2
CO2 + H2O
Balancing
9.6 9.6.1 Cracking
9.6.2 C12O26 → C7H16 + C3H6 + C2H4
Total: 200 marks
IEB Copyright © 2022
You might also like
- CAPE Chemistry Unit 2 Paper 2 2017 AnswersDocument12 pagesCAPE Chemistry Unit 2 Paper 2 2017 Answersemanuel coates100% (9)
- CHM271 - FRONT COVER LAB REPORT (1) - MergedDocument18 pagesCHM271 - FRONT COVER LAB REPORT (1) - Mergednurain huzaineNo ratings yet
- Corrosion-Resistant High-Silicon Iron Castings: Standard Specification ForDocument5 pagesCorrosion-Resistant High-Silicon Iron Castings: Standard Specification Forrobert gridleyNo ratings yet
- AngloGold Fire Assay Training ManualDocument42 pagesAngloGold Fire Assay Training ManualKenneth OrtizNo ratings yet
- FerroceneDocument20 pagesFerroceneKalfakNo ratings yet
- Chemistry Booklet No 4 EngineeringDocument382 pagesChemistry Booklet No 4 EngineeringVarad BhosaleNo ratings yet
- Universiti Teknologi Mara Final Examination: Principles of Physical ChemistryDocument5 pagesUniversiti Teknologi Mara Final Examination: Principles of Physical ChemistryliliNo ratings yet
- 163Ch11 13Document7 pages163Ch11 13Aaron BautistaNo ratings yet
- OCR - Chemistry - Module 5 Part 1 - GraspIT ANSWERS - A LevelDocument10 pagesOCR - Chemistry - Module 5 Part 1 - GraspIT ANSWERS - A LevelSigourney MarshNo ratings yet
- SHCC - 2023 - Chem Paper2 - AnnaDocument8 pagesSHCC - 2023 - Chem Paper2 - AnnaOof GucciNo ratings yet
- 25th Chemistry Shift 2Document13 pages25th Chemistry Shift 2Vaid KulkarniNo ratings yet
- Rate of ReactionDocument44 pagesRate of Reactionpokyik cheungNo ratings yet
- Tutorial-Manual CH1002Document18 pagesTutorial-Manual CH1002Gift Chulu100% (2)
- Sample Paper Chemistry Clas Xi Set 5Document9 pagesSample Paper Chemistry Clas Xi Set 5abhijeetkumar12345trNo ratings yet
- A Level Chemistry Paper 1 Set 12marking GuideDocument17 pagesA Level Chemistry Paper 1 Set 12marking Guidebuuleivan8No ratings yet
- 2021 Chem Skills SolutionsDocument10 pages2021 Chem Skills SolutionsVictor GuanNo ratings yet
- Sk0014 Physical & Iinorganic Chemistry Tutorial 4: Reaction KineticsDocument2 pagesSk0014 Physical & Iinorganic Chemistry Tutorial 4: Reaction KineticsNeil8353 GgNo ratings yet
- NYJC 2021 H2 Chemistry 9729 P1Document14 pagesNYJC 2021 H2 Chemistry 9729 P1Allison KhooNo ratings yet
- 3 EquilibriumDocument44 pages3 EquilibriumEugene ChaiNo ratings yet
- Chm271 - Tutorial 5 - Chemical KineticsDocument6 pagesChm271 - Tutorial 5 - Chemical Kineticsfiefy zmrNo ratings yet
- A Level Chemistry Paper 1 Set 6 Marking GuideDocument19 pagesA Level Chemistry Paper 1 Set 6 Marking GuideSamuel TusubiraNo ratings yet
- Report 5Document8 pagesReport 5Anh HoàngNo ratings yet
- INC150X FISA Paper 2018Document6 pagesINC150X FISA Paper 2018Stolo SbaeNo ratings yet
- SCIENCE Test and KeyDocument15 pagesSCIENCE Test and KeyAlexander StellyNo ratings yet
- PHYS CE Tutorial QuestionsDocument3 pagesPHYS CE Tutorial QuestionsMel SalazarNo ratings yet
- CHM3103 Lab Experiment 2Document15 pagesCHM3103 Lab Experiment 2husnaNo ratings yet
- CHM271 July 2021Document6 pagesCHM271 July 2021aliahhafiz0401No ratings yet
- Chemical Kinetics: Joshua Micole Felizarta, LPTDocument25 pagesChemical Kinetics: Joshua Micole Felizarta, LPTArvin SayotoNo ratings yet
- Class 12 ChemistryDocument10 pagesClass 12 ChemistryDHRUV goswamiNo ratings yet
- HKDSE Chemistry: Suggested Answer For Mock Examination 4 (Paper 2)Document5 pagesHKDSE Chemistry: Suggested Answer For Mock Examination 4 (Paper 2)Vinaigrette HeNo ratings yet
- CE 360 Homework 4 55 PointsDocument6 pagesCE 360 Homework 4 55 PointsMuhammad TehseenNo ratings yet
- 2022 HSC Chemistry MGDocument22 pages2022 HSC Chemistry MGazizi5916No ratings yet
- Chemical EquilibriumDocument32 pagesChemical EquilibriumsanjuNo ratings yet
- Act Thrissur +1 Chem KeyDocument6 pagesAct Thrissur +1 Chem KeyYADUKRISHNAN K NAIRNo ratings yet
- Chemical KineticsDocument22 pagesChemical KineticsEleanorNo ratings yet
- Experiment 5: Factors Affecting Reaction Rate: International University, Vietnam National University - HCMC 1Document8 pagesExperiment 5: Factors Affecting Reaction Rate: International University, Vietnam National University - HCMC 1NaHuynJungNo ratings yet
- Ap-Chem Kinetics fr2Document11 pagesAp-Chem Kinetics fr2hylee102594No ratings yet
- Cbse Test Paper-01 CLASS - XII CHEMISTRY (Chemical Kinetics)Document1 pageCbse Test Paper-01 CLASS - XII CHEMISTRY (Chemical Kinetics)Vinoth KumarNo ratings yet
- Chemical Kinetics: Dr. Mohamed FadelDocument52 pagesChemical Kinetics: Dr. Mohamed Fadelعبدالحميد العرفيNo ratings yet
- AIPMT SOLUTIONS 2011 (English)Document35 pagesAIPMT SOLUTIONS 2011 (English)Resonance KotaNo ratings yet
- How Far MSDocument6 pagesHow Far MSsaadNo ratings yet
- Answer All Question: Sk027 / Chapter 3: Reaction Kinetics / Exercise 1Document5 pagesAnswer All Question: Sk027 / Chapter 3: Reaction Kinetics / Exercise 1kjjkimkmkNo ratings yet
- Soal Uts Trk1 - 2013-PagiDocument2 pagesSoal Uts Trk1 - 2013-PagiRifqiMuhammadNo ratings yet
- A Level Chemistry Paper 1 Set 2 Marking GuideDocument7 pagesA Level Chemistry Paper 1 Set 2 Marking Guidessentume peterNo ratings yet
- CBSE Class 12 Question Paper 2019 Chemistry Set 1Document15 pagesCBSE Class 12 Question Paper 2019 Chemistry Set 1Saran.kNo ratings yet
- Le Chatelier's PrincipleDocument18 pagesLe Chatelier's PrincipleMonaliza MandacNo ratings yet
- JEE Main 2022 July Session 2 Shift-1 (DT 27-07-2022) ChemistryDocument11 pagesJEE Main 2022 July Session 2 Shift-1 (DT 27-07-2022) ChemistryResonance EduventuresNo ratings yet
- CIC Exam 2000Document17 pagesCIC Exam 2000Bankai's Derek LeongNo ratings yet
- Class Xii Chem Term Ii SQPDocument32 pagesClass Xii Chem Term Ii SQPAmaan KhanNo ratings yet
- 2015 PSPM Kedah Kimia2 W AnsDocument38 pages2015 PSPM Kedah Kimia2 W Ansjee2kk100% (2)
- KE - Equilibrium Packet 2-2-21Document2 pagesKE - Equilibrium Packet 2-2-21joaseNo ratings yet
- Experiment 3 LabrepDocument10 pagesExperiment 3 LabrepDI LacsonNo ratings yet
- Class Room Problems: Page # 44 Chemical KineticsDocument57 pagesClass Room Problems: Page # 44 Chemical Kineticsd anjilappaNo ratings yet
- Test - 1 QA CHEF 114 - Tri 3 - March 2012 - UPDATED and Finalized 29 FEB 2012 PDFDocument10 pagesTest - 1 QA CHEF 114 - Tri 3 - March 2012 - UPDATED and Finalized 29 FEB 2012 PDFSamsuddin MusaNo ratings yet
- 24th Chemistry Shift 2Document14 pages24th Chemistry Shift 2Vaid KulkarniNo ratings yet
- K01628 - 20191122173524 - Tutorial 1 Sku1023Document1 pageK01628 - 20191122173524 - Tutorial 1 Sku1023venosyah devanNo ratings yet
- ChemyDocument1 pageChemyvenosyah devanNo ratings yet
- Equilibrium 2016Document58 pagesEquilibrium 2016api-546066323No ratings yet
- MCD4390 Week 10 Tutorial QuestionsDocument5 pagesMCD4390 Week 10 Tutorial QuestionsGabbar100% (1)
- Question Paper June 2023 (H43201)Document28 pagesQuestion Paper June 2023 (H43201)Dominic Afoakwah (Jack)No ratings yet
- National Institute of Technology Calicut Department of ChemistryDocument1 pageNational Institute of Technology Calicut Department of ChemistryNanditha ANo ratings yet
- Republic Polytechnic A348 Water and Wastewater Treatment Worksheet 6: Dirt in KettleDocument9 pagesRepublic Polytechnic A348 Water and Wastewater Treatment Worksheet 6: Dirt in KettleLim Liang XuanNo ratings yet
- On Solar Hydrogen and NanotechnologyFrom EverandOn Solar Hydrogen and NanotechnologyLionel VayssieresNo ratings yet
- AEROSIL® 150 - EvonikDocument2 pagesAEROSIL® 150 - EvonikSafiullah KhanNo ratings yet
- Plating Au Cu Ni Ag PhotoresistsDocument11 pagesPlating Au Cu Ni Ag Photoresistsjayaprakash reddyNo ratings yet
- Null 1Document7 pagesNull 1Yuva YuvarajNo ratings yet
- Dapus Rekristalisasi.Document35 pagesDapus Rekristalisasi.RamaNo ratings yet
- 10.2 Grignard 反応と関連反応: Mg Et O 1) 2) H SO /H ODocument8 pages10.2 Grignard 反応と関連反応: Mg Et O 1) 2) H SO /H OHiman KumarNo ratings yet
- Latihan PDPR Kimia SPM 2021 Pada 21 AprilDocument8 pagesLatihan PDPR Kimia SPM 2021 Pada 21 Aprilexo kaihunNo ratings yet
- Project Proposal DocumentDocument5 pagesProject Proposal DocumentInnocent PunungweNo ratings yet
- Conversion of Waste Cooking Oil To Biodiesel: Chemistry Department, SMDRSD College Pathankot, 145001, IndiaDocument14 pagesConversion of Waste Cooking Oil To Biodiesel: Chemistry Department, SMDRSD College Pathankot, 145001, Indiaferry merryNo ratings yet
- Role of Iron Fe in BodyDocument9 pagesRole of Iron Fe in Bodymuntada3000.mkNo ratings yet
- Large-Scale Dynamic Simulation For Security Assessment of An Ethylene Oxide Manufacturing Process PDFDocument17 pagesLarge-Scale Dynamic Simulation For Security Assessment of An Ethylene Oxide Manufacturing Process PDFMary B.No ratings yet
- Liquid Organic Hydrogen CarriersDocument8 pagesLiquid Organic Hydrogen CarriersGrad OanaNo ratings yet
- Corrosion and Its ControlDocument29 pagesCorrosion and Its Controlomer faruqeNo ratings yet
- Alchemy: by Kenneth HiteDocument12 pagesAlchemy: by Kenneth HiteDraquarNo ratings yet
- 2026 Sulfuric Acid Analyzer: From Metrohm Process AnalyticsDocument1 page2026 Sulfuric Acid Analyzer: From Metrohm Process AnalyticspinitNo ratings yet
- Science (Module 1 - Lessons 1-4) : Summative TestDocument4 pagesScience (Module 1 - Lessons 1-4) : Summative TestAngelica BuquiranNo ratings yet
- Determinación de Acrilamida en Dieferentes MaterialesDocument88 pagesDeterminación de Acrilamida en Dieferentes MaterialesMarianella ZegarraNo ratings yet
- Activity 12 Analysis of BloodDocument2 pagesActivity 12 Analysis of BloodLiane BartolomeNo ratings yet
- 17 - 18 Carbon Cycle - Humus Formation PDFDocument10 pages17 - 18 Carbon Cycle - Humus Formation PDFshubhamNo ratings yet
- Recent Developments in The Synthesis of Prostaglandins and AnaloguesDocument52 pagesRecent Developments in The Synthesis of Prostaglandins and AnaloguesaliNo ratings yet
- Chemstry June 2001 - Paper 1Document17 pagesChemstry June 2001 - Paper 1theyaasir100% (2)
- 2012 Chemetrics CTLGDocument91 pages2012 Chemetrics CTLGFamc CmafNo ratings yet
- InsulationDocument10 pagesInsulationJyotiNo ratings yet
- 2017-GCWUF-3350 SUMAIRA SAIF (Roll# 189) Final Assignment (1) Inorganic ChemistDocument5 pages2017-GCWUF-3350 SUMAIRA SAIF (Roll# 189) Final Assignment (1) Inorganic ChemistSumaira SaifNo ratings yet
- BTLGT Day1Document104 pagesBTLGT Day1John Walter EstradaNo ratings yet
- Chrome Yellow ExperimentDocument3 pagesChrome Yellow ExperimentAkhil KumarNo ratings yet
- Codex Standard For GoudaDocument5 pagesCodex Standard For GoudaTarek ShaheenNo ratings yet
- The Potentials of Henna (L) Leaves Extracts As Counter Stain in Gram Staining ReactionDocument5 pagesThe Potentials of Henna (L) Leaves Extracts As Counter Stain in Gram Staining ReactionAna Virginia MontoyaNo ratings yet