Professional Documents
Culture Documents
Coordination Compounds - Short Notes - Prayas JEE 2.0 2024
Uploaded by
aryasushama2611Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Coordination Compounds - Short Notes - Prayas JEE 2.0 2024
Uploaded by
aryasushama2611Copyright:
Available Formats
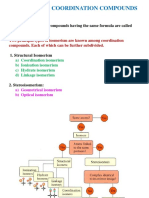
CHAPTER
11 Coordination Compounds
Representation of Complex Compound Exception
ligand
d3s hybridisation, Td, diamagnetic, inner orbital complex eg.
co-ordination n± charge on
MLX coordination sphere MnO 4– , CrO 2– 2– 3–
sphere 4 , Cr2 O 7 , CrO 2 Cl 2 , CrO 2 F2 , VO 4
central metal ion number of ligand Transference of electron
Co-ordination number = Number of atoms surrounded to eg. Cu+2 in C.N. → 4 with L
central metal ion. -
Notes: (where L = NO 2 / CN– / NH3 etc.)
Bidentate and Polydentate are also called chelating ligand. Organometallic Compounds
Bonding in Coordination Compound Compounds in which the less E.N. (Ge, Sb, B, Si, P, As) central
Effective Atomic Number & Sidgwick Rule metal atoms are bonded directly to carbon atoms are called
Total number of electron present on central metal atom or ion organometallic compounds.
after accepting the electron pair from ligand.
s-bonded compounds formed by nontransition elements.
VBT R-Mg-X, (CH3–CH2)2 Zn, Ziegler natta catalyst, etc.
Metal provoide hybridised vacant orbital for the acceptance
of lone pair from ligand. p-bonded organometallic compounds are generally formed
Hybridisation, shape and magnetic behaviour of complex by transition elements e.g. Zeise’s salt, ferrocene, dibenzene
depends upon the nature of ligand. chromium, etc.
Strong field ligand pair up the unpaired e– of central metal s-and p-bonded organometallic compounds: Metal carbonyls
atom where as weak field ligand does not.
If unpaired e– present in complex then complex is compounds formed between metal and carbon monoxide
paramagnetic. If unpaired e– is absent then diamagnatic. belong to this class. Ni(CO)4, Fe(CO)5 etc.
eg. C.N. = 4; [NiCl4]2–
Stereo Isomerism
Series which shows the Relative Strength of Ligands
I– (weakest) < Br– < SCN– < Cl– < S2– < F– < OH– < C2O42– < H2O Stereo Isomerism in Co-ordination Compound
< NCS– < edta4– < NH3 < en < CN– < CO(strongest) CN-4
Exception Square planar complex does not show optical isomerism.
[Co(OX)3]3– d2sp3 diamagnetic Square planar complex show optical activity if the co-ordinated
ligand having chiral center.
[Co(H2O)6]3+ d2sp3 diamagnetic
Square planar complex
[NiF6]2– d2sp3 diamagnetic [Ma2b2]n± , [Ma2bc]n± , [Mabcd]n± , [M(AB)cd]n±
[Cr(NH3)6 ]2+ sp3d2 paramagnetic [M(AB)(CD)]n± show geometrical isomerism
[Mn(NH3)6]2+ sp3d2 paramagnetic [Mabcd]n± form two cis and one trans.
[Fe(NH3)6]2+ sp3d2 paramagnetic Tetrahedral complex [Mabcd]n±, [M(AB)cd]n± [M(AB)(CD)] n±
show optical isomerism
[CoL6]4– (L = NO2– /CN–) d2sp3 paramagnatic
Tetrahdral complex does not show geometrical isomerism.
You might also like
- Periodic Trends Atomic RadiusDocument6 pagesPeriodic Trends Atomic RadiusGabriel TaylorNo ratings yet
- D-Block Metal Chemistry: General ConsiderationsDocument23 pagesD-Block Metal Chemistry: General ConsiderationsPrativa BeheraNo ratings yet
- New Module-2 Inorganic and Organometallic Chem Fall-2023Document67 pagesNew Module-2 Inorganic and Organometallic Chem Fall-2023VICHUNo ratings yet
- Revision Notes On Co-Ordination CompoundsDocument12 pagesRevision Notes On Co-Ordination CompoundsAnonymous vRpzQ2BLNo ratings yet
- Topic 8 - Coordination CompoundDocument40 pagesTopic 8 - Coordination Compoundizz isalahNo ratings yet
- Chemistry Module 2 Part 2Document60 pagesChemistry Module 2 Part 2RiyazNo ratings yet
- Gen Inorg Chem09 10 PDFDocument26 pagesGen Inorg Chem09 10 PDFDebdeep RayNo ratings yet
- Module-2-Dr RKDocument55 pagesModule-2-Dr RKIshaan SawantNo ratings yet
- Master Card - Coordination CompoundsDocument2 pagesMaster Card - Coordination CompoundsgudiNo ratings yet
- Isomer Dan Spektrokimia Ok 2017Document90 pagesIsomer Dan Spektrokimia Ok 2017joyoNo ratings yet
- Coordination CompoundsDocument51 pagesCoordination CompoundsasdfNo ratings yet
- Inorganic Chapter19Document23 pagesInorganic Chapter19barkatullah0% (1)
- Theory EnglishDocument11 pagesTheory Englishd anjilappaNo ratings yet
- Chemistry Module 2 Application If Metal ComplexesDocument56 pagesChemistry Module 2 Application If Metal ComplexesRiyazNo ratings yet
- Transition Metals and Coordination ChemistryDocument77 pagesTransition Metals and Coordination ChemistryAdistaNo ratings yet
- Co-Ordination ChemistryDocument27 pagesCo-Ordination Chemistrymdsaadr856No ratings yet
- UNIT 9 Topic: Coordination CompoundsDocument9 pagesUNIT 9 Topic: Coordination CompoundsDeva RajNo ratings yet
- Problem Xii emDocument34 pagesProblem Xii emAjayNo ratings yet
- CY 101coordination Compounds1Document121 pagesCY 101coordination Compounds1Mukul SuryawanshiNo ratings yet
- Chemistry NotesDocument5 pagesChemistry Notesparth PatelNo ratings yet
- Simplified Focus Area Notes Ii CorrDocument8 pagesSimplified Focus Area Notes Ii Corrwargod RAMZNo ratings yet
- Coordinate CompoundsDocument5 pagesCoordinate Compoundsmandhareneel06No ratings yet
- CFT and Chelate Effect-IDocument65 pagesCFT and Chelate Effect-IHitesh vadherNo ratings yet
- Module 2 - Metal Complexes and OrganometallicsDocument55 pagesModule 2 - Metal Complexes and Organometallicstaara022006No ratings yet
- Magnetochemie SeminarzumPraktikum 2012Document25 pagesMagnetochemie SeminarzumPraktikum 2012SANKAR VNo ratings yet
- N4. D-Block Elements (HL)Document13 pagesN4. D-Block Elements (HL)Yuvraj GuptaNo ratings yet
- Transition Elements PDFDocument18 pagesTransition Elements PDFArslanAliNo ratings yet
- D-Block Elements - DTS 1 SolDocument2 pagesD-Block Elements - DTS 1 SolRudra guptaNo ratings yet
- Inorganic Reaction Mechanisms: January 2020Document225 pagesInorganic Reaction Mechanisms: January 2020AdistaNo ratings yet
- Coordinate ChemistryDocument15 pagesCoordinate ChemistryJinal VadodariyaNo ratings yet
- Chapter 9 Co Ordination CompoundsDocument46 pagesChapter 9 Co Ordination Compoundssukaina fatimaNo ratings yet
- Coordination ChemistryDocument10 pagesCoordination ChemistrycharleslukeNo ratings yet
- Chem Chap 5 Coordination CompoundsDocument71 pagesChem Chap 5 Coordination Compoundsissacpaul382No ratings yet
- Transition Metals PDFDocument30 pagesTransition Metals PDFMaheshNo ratings yet
- Notes Group TEDocument6 pagesNotes Group TEThilagaNo ratings yet
- D and F - Block Elements in NutshellDocument14 pagesD and F - Block Elements in NutshellPrem MehrotraNo ratings yet
- Coordination CompdsDocument7 pagesCoordination CompdsAnil BahriNo ratings yet
- Coordination ComplexesDocument33 pagesCoordination ComplexesMashai LesenyehoNo ratings yet
- Xii - CH9 - Coordination CompoundsDocument8 pagesXii - CH9 - Coordination CompoundsYash RajNo ratings yet
- Chapter - 09: Coordination CompoundsDocument30 pagesChapter - 09: Coordination CompoundsTeju tejasNo ratings yet
- Dr. A. A. Akinsiku: Selected Topics in Chemistry For Chemical Engineering 1 BYDocument63 pagesDr. A. A. Akinsiku: Selected Topics in Chemistry For Chemical Engineering 1 BYIfiok UsoroNo ratings yet
- 12 Chemistry Impq CH07 The P Block Elements 03Document15 pages12 Chemistry Impq CH07 The P Block Elements 03Ricky SharmaNo ratings yet
- MOT (Contd) Valence Bond Theory - Dr. Akinsiku A. A.Document13 pagesMOT (Contd) Valence Bond Theory - Dr. Akinsiku A. A.Ifiok UsoroNo ratings yet
- Ms ChauhanDocument4 pagesMs ChauhanNikhil VarshneyNo ratings yet
- ch-9 ExerciseDocument31 pagesch-9 ExerciseTr Mazhar PunjabiNo ratings yet
- Part Vi Stabilization, Kinetics&thermodyamics of ComplexesDocument32 pagesPart Vi Stabilization, Kinetics&thermodyamics of ComplexesJohn QambeshNo ratings yet
- VBT - Theory and IsomerismDocument25 pagesVBT - Theory and Isomerismzuneid-adamoNo ratings yet
- Module-2:: Metal Complexes and OrganometallicsDocument75 pagesModule-2:: Metal Complexes and OrganometallicsAshutosh100% (2)
- Coordination Chemistry:: An OverviewDocument37 pagesCoordination Chemistry:: An OverviewAnmol KalantriNo ratings yet
- Chem NotesDocument17 pagesChem NotesCND PurPNo ratings yet
- An Introduction To Transition Metal ChemistryDocument41 pagesAn Introduction To Transition Metal ChemistryPari GandepalliNo ratings yet
- Did We Forget Square Planar Complexes?Document34 pagesDid We Forget Square Planar Complexes?Shubham KumarNo ratings yet
- Complexometric Titrations: 3 Year Students, General-ScienceDocument50 pagesComplexometric Titrations: 3 Year Students, General-ScienceHesham AlsoghierNo ratings yet
- Notes: T.me/anandmaniDocument41 pagesNotes: T.me/anandmaniIshanshu BajpaiNo ratings yet
- Chapter 9 Coordination CompoundsDocument10 pagesChapter 9 Coordination CompoundsDipti GuptaNo ratings yet
- Coordination ChemistryDocument76 pagesCoordination ChemistryLipsa PradhanNo ratings yet
- Coordination Compounds 2Document48 pagesCoordination Compounds 2pavithra KumarNo ratings yet
- Hsslive Xii Chemistry CH 9 Coordination Compounds by SajeevDocument2 pagesHsslive Xii Chemistry CH 9 Coordination Compounds by SajeevrasalgafoorrvgNo ratings yet
- Coordination CompoundsDocument97 pagesCoordination CompoundsAnant SharmaNo ratings yet
- D-Block Metal Chem.Document26 pagesD-Block Metal Chem.MaheshNo ratings yet
- Nut Astm A563M 10S Bolt/Screw Astm A325M-1: Proof ST Proof LDocument1 pageNut Astm A563M 10S Bolt/Screw Astm A325M-1: Proof ST Proof LDebulus PR0% (1)
- Science 8 3rd Grading ExamDocument3 pagesScience 8 3rd Grading ExamJon Mitchel GalangNo ratings yet
- Thermite WeldingDocument21 pagesThermite WeldingNidhi SharmaNo ratings yet
- ASME P NumbersDocument3 pagesASME P Numbersrajesh100% (1)
- ICSE Selina Solution For Class 9 Chemistry Chapter 2Document13 pagesICSE Selina Solution For Class 9 Chemistry Chapter 2ABHISHEK THAKURNo ratings yet
- Chemical Compatibility Bronce AcidoDocument1 pageChemical Compatibility Bronce AcidoOliver Quezada InostrozaNo ratings yet
- Chemistry Reviewer Part 1Document3 pagesChemistry Reviewer Part 1kurtbusbus1No ratings yet
- ASTM B275-05 Codification of Certain Metals and AlloysDocument7 pagesASTM B275-05 Codification of Certain Metals and AlloysDemian LópezNo ratings yet
- Stainless Steels Welding Guide - LINCOLN-ELECTRICDocument40 pagesStainless Steels Welding Guide - LINCOLN-ELECTRICFrancisco Xavier Cabrera LozaNo ratings yet
- Solubility of Compounds in WaterDocument2 pagesSolubility of Compounds in WaterShiann SampleNo ratings yet
- 2019 Al Chemistry Part I MCQ Paper New Syllabus Sinhala Medium Alevelapi PDFDocument9 pages2019 Al Chemistry Part I MCQ Paper New Syllabus Sinhala Medium Alevelapi PDFsavindu pereraNo ratings yet
- Chemical Analyses-Stainless Steels Duplex and Special AlloysDocument8 pagesChemical Analyses-Stainless Steels Duplex and Special AlloysStuartNo ratings yet
- Hydrogen Key NotesDocument4 pagesHydrogen Key NotesalishNo ratings yet
- Effect of Alloying ElementsDocument86 pagesEffect of Alloying ElementsNavdeep JainNo ratings yet
- Valency TableDocument2 pagesValency TableZarbEChishtiNo ratings yet
- Divya Ganeshwala 8. Chemistry Investigatory ProjectDocument11 pagesDivya Ganeshwala 8. Chemistry Investigatory ProjectChetan Suhas KamtheNo ratings yet
- 0620 s08 QP 3 2Document25 pages0620 s08 QP 3 2Varun PanickerNo ratings yet
- 4CH0 1CR Que 20130520Document36 pages4CH0 1CR Que 20130520Rahil Tasawar100% (1)
- Cambridge IGCSE: Chemistry 0620/22Document16 pagesCambridge IGCSE: Chemistry 0620/22hhheeeNo ratings yet
- Electrolysis 3 MSDocument14 pagesElectrolysis 3 MSEnoch AdebiyiNo ratings yet
- Catholic Junior College: JC1 Mid-Year Examinations Higher 2Document8 pagesCatholic Junior College: JC1 Mid-Year Examinations Higher 2Timothy HandokoNo ratings yet
- Reactivity Series - Reactions of Metals Summaried Into A Table PDFDocument1 pageReactivity Series - Reactions of Metals Summaried Into A Table PDFVictoria KairooNo ratings yet
- HCL PDFDocument13 pagesHCL PDFjoycepeterNo ratings yet
- D and F PW New ModuDocument32 pagesD and F PW New ModuIshant SankhalaNo ratings yet
- Laboratory PreparationsDocument15 pagesLaboratory PreparationsMinn ThantNo ratings yet
- Chemistry - Revision - Grade - 8 - 1st - Term Answer KeyDocument9 pagesChemistry - Revision - Grade - 8 - 1st - Term Answer KeyWaLkiEs TaLkIeSNo ratings yet
- KSSM Form 1 Science Chapter 6Document3 pagesKSSM Form 1 Science Chapter 6elizabeth ellsaNo ratings yet
- TRADEJINI MCX Margin File For 16/07/2021: BullionsDocument3 pagesTRADEJINI MCX Margin File For 16/07/2021: BullionsmavenNo ratings yet
- Tugas Kimter Pak EdwinDocument34 pagesTugas Kimter Pak EdwinaudheaykNo ratings yet