Professional Documents
Culture Documents
2010 Checklist
Uploaded by
muhammed barznjiCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
2010 Checklist
Uploaded by
muhammed barznjiCopyright:
Available Formats
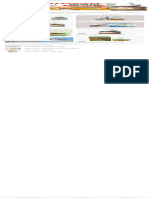
CONSORT 2010 checklist of information to include when reporting a randomised trial *
Item Reported
Section/Topic No Checklist item on page No
Title and abstract
1a Identification as a randomised trial in the title
1b Structured summary of trial design, methods, results, and conclusions (for specific guidance see CONSORT for abstracts)
Introduction
Background and 2a Scientific background and explanation of rationale
objectives 2b Specific objectives or hypotheses
Methods
Trial design 3a Description of trial design (such as parallel, factorial) including allocation ratio
3b Important changes to methods after trial commencement (such as eligibility criteria), with reasons
Participants 4a Eligibility criteria for participants
4b Settings and locations where the data were collected
Interventions 5 The interventions for each group with sufficient details to allow replication, including how and when they were
actually administered
Outcomes 6a Completely defined pre-specified primary and secondary outcome measures, including how and when they
were assessed
6b Any changes to trial outcomes after the trial commenced, with reasons
Sample size 7a How sample size was determined
7b When applicable, explanation of any interim analyses and stopping guidelines
Randomisation:
Sequence 8a Method used to generate the random allocation sequence
generation 8b Type of randomisation; details of any restriction (such as blocking and block size)
Allocation 9 Mechanism used to implement the random allocation sequence (such as sequentially numbered containers),
concealment describing any steps taken to conceal the sequence until interventions were assigned
mechanism
Implementation 10 Who generated the random allocation sequence, who enrolled participants, and who assigned participants to
interventions
Blinding 11a If done, who was blinded after assignment to interventions (for example, participants, care providers, those
CONSORT 2010 checklist Page 1
assessing outcomes) and how
11b If relevant, description of the similarity of interventions
Statistical methods 12a Statistical methods used to compare groups for primary and secondary outcomes
12b Methods for additional analyses, such as subgroup analyses and adjusted analyses
Results
Participant flow (a 13a For each group, the numbers of participants who were randomly assigned, received intended treatment, and
diagram is strongly were analysed for the primary outcome
recommended) 13b For each group, losses and exclusions after randomisation, together with reasons
Recruitment 14a Dates defining the periods of recruitment and follow-up
14b Why the trial ended or was stopped
Baseline data 15 A table showing baseline demographic and clinical characteristics for each group
Numbers analysed 16 For each group, number of participants (denominator) included in each analysis and whether the analysis was
by original assigned groups
Outcomes and 17a For each primary and secondary outcome, results for each group, and the estimated effect size and its
estimation precision (such as 95% confidence interval)
17b For binary outcomes, presentation of both absolute and relative effect sizes is recommended
Ancillary analyses 18 Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing
pre-specified from exploratory
Harms 19 All important harms or unintended effects in each group (for specific guidance see CONSORT for harms)
Discussion
Limitations 20 Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses
Generalisability 21 Generalisability (external validity, applicability) of the trial findings
Interpretation 22 Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence
Other information
Registration 23 Registration number and name of trial registry
Protocol 24 Where the full trial protocol can be accessed, if available
Funding 25 Sources of funding and other support (such as supply of drugs), role of funders
Citation: Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Medicine. 2010;8:18.
© 2010 Schulz et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits
unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
*We strongly recommend reading this statement in conjunction with the CONSORT 2010 Explanation and Elaboration for important clarifications on all the items. If relevant, we also recommend
reading CONSORT extensions for cluster randomised trials, non-inferiority and equivalence trials, non-pharmacological treatments, herbal interventions, and pragmatic trials. Additional
extensions are forthcoming: for those and for up-to-date references relevant to this checklist, see www.consort-statement.org.
CONSORT 2010 checklist Page 2
You might also like
- JSA For Excavation of Fiber Optical Cable WR-501 To WR-617 Community To Petro Rabigh......Document15 pagesJSA For Excavation of Fiber Optical Cable WR-501 To WR-617 Community To Petro Rabigh......Habib ur rahmanNo ratings yet
- Wais IV Interpretation GuideDocument13 pagesWais IV Interpretation GuideRemik BuczekNo ratings yet
- Documentation of Clinical Trial Monitoring: A practical guide compliant with Good Clinical PracticeFrom EverandDocumentation of Clinical Trial Monitoring: A practical guide compliant with Good Clinical PracticeRating: 5 out of 5 stars5/5 (1)
- 2011 YFBCA Youth Football Coaches Exam: Making Good Coaches, Great Teachers!Document10 pages2011 YFBCA Youth Football Coaches Exam: Making Good Coaches, Great Teachers!Coach Joe BouffardNo ratings yet
- Discrimination Testing in Sensory Science: A Practical HandbookFrom EverandDiscrimination Testing in Sensory Science: A Practical HandbookRating: 3 out of 5 stars3/5 (2)
- Consort 2010 Checklist for Reporting Randomized TrialsDocument2 pagesConsort 2010 Checklist for Reporting Randomized Trialsziana alawiyah75% (4)
- CONSORT 2010 ChecklistDocument2 pagesCONSORT 2010 ChecklistReza Ishak EstikoNo ratings yet
- Cheklist 2016Document3 pagesCheklist 2016Ida Yustina FalahiNo ratings yet
- Auinst CHKDocument1 pageAuinst CHKDr.Niveditha SNo ratings yet
- Form Checklist CONSORTDocument3 pagesForm Checklist CONSORTHolmes MoryNo ratings yet
- Consort TableDocument3 pagesConsort TablepubdokcrssNo ratings yet
- ResearchMethodologyChecklistsFull PDFDocument52 pagesResearchMethodologyChecklistsFull PDFvenkateshNo ratings yet
- Consort ChartDocument2 pagesConsort ChartSafira RamadhaniNo ratings yet
- CONSORT 2010 ChecklistDocument6 pagesCONSORT 2010 Checklistfaridah azzah sariNo ratings yet
- 2964 CONSORT+2010+ChecklistDocument3 pages2964 CONSORT+2010+ChecklistRina WatiNo ratings yet
- Ecommended Items To Address in A Clinical Trial Protocol and Related DocumentsDocument5 pagesEcommended Items To Address in A Clinical Trial Protocol and Related DocumentsIoana LeseNo ratings yet
- SPIRIT Checklist Download 8jan13Document5 pagesSPIRIT Checklist Download 8jan13gianpoerNo ratings yet
- Jurnal Cluster Randomised CT Dan CONSORT (Shelly, Yuliarni, Anita, Dwi Mayang, Dicky, Alamsyah)Document11 pagesJurnal Cluster Randomised CT Dan CONSORT (Shelly, Yuliarni, Anita, Dwi Mayang, Dicky, Alamsyah)shelly juliskaNo ratings yet
- 2964 CONSORT+2010+ChecklistDocument3 pages2964 CONSORT+2010+ChecklistNur UlayatilmiladiyyahNo ratings yet
- CONSORT 2010 Checklist1Document6 pagesCONSORT 2010 Checklist1faridah azzah sariNo ratings yet
- Consort ChecklistDocument2 pagesConsort ChecklistAyuAnatrieraNo ratings yet
- CONSORT Statement 2001 Checklist: Items To Include When Reporting A Randomized TrialDocument1 pageCONSORT Statement 2001 Checklist: Items To Include When Reporting A Randomized TrialΠόπη ΜποζίκηNo ratings yet
- CONSORT 2010 checklist of information to include when reporting a randomised trialDocument3 pagesCONSORT 2010 checklist of information to include when reporting a randomised trialSri WahyudiNo ratings yet
- Critical Appraisal of Journal Article - 5: BY DR - Gautam.G.J, Third Year PostgraduateDocument36 pagesCritical Appraisal of Journal Article - 5: BY DR - Gautam.G.J, Third Year PostgraduateGautam G JNo ratings yet
- Apraisal Jurnal-Westi Permata Wati-S2 Pendidikan Kedokteran 2022Document8 pagesApraisal Jurnal-Westi Permata Wati-S2 Pendidikan Kedokteran 2022yugoNo ratings yet
- CONSORT Checklist of Information To Include When Reporting A Randomised TrialDocument3 pagesCONSORT Checklist of Information To Include When Reporting A Randomised TrialJoko Tri WahyudiNo ratings yet
- Apraisal Jurnal-Westi Permata Wati-S2 Pendidikan Kedokteran 2022Document8 pagesApraisal Jurnal-Westi Permata Wati-S2 Pendidikan Kedokteran 2022yugoNo ratings yet
- Penerapan CONSORT dalam jurnal internasionalDocument4 pagesPenerapan CONSORT dalam jurnal internasionalrisdaseptianaNo ratings yet
- 2965 CONSORT+2010+ChecklistDocument2 pages2965 CONSORT+2010+ChecklistsongsiriNo ratings yet
- SPIRIT Checklist12Document9 pagesSPIRIT Checklist12Kamylla Caroline SantosNo ratings yet
- ChecklistDocument6 pagesChecklistpedstrickles1No ratings yet
- CONSORT ChecklistDocument3 pagesCONSORT Checklistkitty motoNo ratings yet
- Text S1 - Checklist of Items To Include When Reporting A Systematic Review or Meta-AnalysisDocument3 pagesText S1 - Checklist of Items To Include When Reporting A Systematic Review or Meta-AnalysiseDy TrujilloNo ratings yet
- 2009 Checklist: Section/topic # Checklist Item Reported On Page #Document2 pages2009 Checklist: Section/topic # Checklist Item Reported On Page #kfctcoNo ratings yet
- The CONSORT Statement: ArticleDocument4 pagesThe CONSORT Statement: ArticleiikhsanhNo ratings yet
- PRISMA 2009 Checklist PDFDocument2 pagesPRISMA 2009 Checklist PDFSunisa KhamkeawNo ratings yet
- Critical Appraisal ToolsDocument8 pagesCritical Appraisal ToolsPurwanti ArianiNo ratings yet
- 3.2.16.highland Emergency Medicine Journal Club Methods.2Document2 pages3.2.16.highland Emergency Medicine Journal Club Methods.2andrew herringNo ratings yet
- CONSORT 2010 Checklist of Information To Include When Reporting A Randomised TrialDocument2 pagesCONSORT 2010 Checklist of Information To Include When Reporting A Randomised TrialIntan Winta PratiwiNo ratings yet
- PRISMA checklist reviewDocument17 pagesPRISMA checklist reviewwendihiNo ratings yet
- S2 Table PRISMA Checklist: TitleDocument2 pagesS2 Table PRISMA Checklist: TitlemirelaNo ratings yet
- 2964 CONSORT+2010+ChecklistDocument3 pages2964 CONSORT+2010+ChecklistontakecilNo ratings yet
- Table S1: PRISMA 2009 Checklist: TitleDocument2 pagesTable S1: PRISMA 2009 Checklist: TitleHirbo ShoreNo ratings yet
- Centang Ebm Siap PrintDocument2 pagesCentang Ebm Siap PrintAmalia Ralita LanuruNo ratings yet
- CONSORT 2010 Checklist of Information To Include When Reporting A Randomised TrialDocument2 pagesCONSORT 2010 Checklist of Information To Include When Reporting A Randomised TrialAnonymous bbLNsOpAEINo ratings yet
- PRISMA 2009 Checklist: An AnalysisDocument2 pagesPRISMA 2009 Checklist: An AnalysisFiŗåš ÀßßâşNo ratings yet
- CRISChecklistfor Reporting Invitro StudiesDocument2 pagesCRISChecklistfor Reporting Invitro StudiesErika Vega0% (1)
- AGREE Reporting ChecklistDocument5 pagesAGREE Reporting ChecklistAngyP0% (1)
- TRIPOD Checklist: Prediction Model Development: Section/Topic Item Checklist Item Title and AbstractDocument1 pageTRIPOD Checklist: Prediction Model Development: Section/Topic Item Checklist Item Title and AbstractA.No ratings yet
- Tripod Checlist Prediction Model Development WordDocument1 pageTripod Checlist Prediction Model Development WordGeovany Herrera ghNo ratings yet
- PRISMA 2009 ChecklistDocument2 pagesPRISMA 2009 ChecklistMelina RamosNo ratings yet
- STROBE Checklist Case-ControlDocument2 pagesSTROBE Checklist Case-ControlAriana OroscoNo ratings yet
- Strobe Cohort Cross Sectional Case ControlDocument3 pagesStrobe Cohort Cross Sectional Case ControlOvanRamadhaNo ratings yet
- CONSORT Group CONSORT 2010 Statement Updated GuideDocument8 pagesCONSORT Group CONSORT 2010 Statement Updated GuideSmulskii AndriiNo ratings yet
- Text S1. PRISMA 2009 Checklist: TitleDocument2 pagesText S1. PRISMA 2009 Checklist: TitleJonathan WelchNo ratings yet
- STROBE Statement-Checklist of Items That Should Be Included in Reports of Observational StudiesDocument3 pagesSTROBE Statement-Checklist of Items That Should Be Included in Reports of Observational StudiesSiti RahmaNo ratings yet
- STROBE Statement-Checklist of Items That Should Be Included in Reports of Observational StudiesDocument3 pagesSTROBE Statement-Checklist of Items That Should Be Included in Reports of Observational StudiesSiti RahmaNo ratings yet
- Guidelines for Reporting Health Research: A User's ManualFrom EverandGuidelines for Reporting Health Research: A User's ManualDavid MoherNo ratings yet
- Paralytic ileus and small intestine 3 copy copyDocument43 pagesParalytic ileus and small intestine 3 copy copymuhammed barznjiNo ratings yet
- NasalDocument1 pageNasalmuhammed barznjiNo ratings yet
- Fsurg 08 730261Document6 pagesFsurg 08 730261muhammed barznjiNo ratings yet
- 43 JMSCRDocument5 pages43 JMSCRmuhammed barznjiNo ratings yet
- Medip, ISJ-8954 ODocument5 pagesMedip, ISJ-8954 Omuhammed barznjiNo ratings yet
- Post Operative Intravenous Fluids: Supervised By: DR Dilan ZakariaDocument49 pagesPost Operative Intravenous Fluids: Supervised By: DR Dilan Zakariamuhammed barznjiNo ratings yet
- COVID-19: Breaking Dawn: Out of The Darkness Comes The LightDocument2 pagesCOVID-19: Breaking Dawn: Out of The Darkness Comes The Lightmuhammed barznjiNo ratings yet
- Monograph IV Cefazolin PDFDocument3 pagesMonograph IV Cefazolin PDFmuhammed barznjiNo ratings yet
- 500 SBA Gen Systemic Pathology PDFDocument27 pages500 SBA Gen Systemic Pathology PDFSanj.etcNo ratings yet
- Drug Treatment for COVID-19: Quick Summary for PCPsDocument3 pagesDrug Treatment for COVID-19: Quick Summary for PCPsmuhammed barznjiNo ratings yet
- Piis0140673620305390 PDFDocument10 pagesPiis0140673620305390 PDFmuhammed barznjiNo ratings yet
- Guidelines For Burial and Safe Management of COVID 19 Dead Body 0803 - MayDocument3 pagesGuidelines For Burial and Safe Management of COVID 19 Dead Body 0803 - MayAsif KhanNo ratings yet
- Nelson Textbook of Pediatrics, 21st Edition 2020 2Document4 pagesNelson Textbook of Pediatrics, 21st Edition 2020 2lj ChuaNo ratings yet
- TUV FS Engineer SISDocument5 pagesTUV FS Engineer SISheikelNo ratings yet
- The Role of Toxicity Testing in Identifying Toxic SubstancesDocument33 pagesThe Role of Toxicity Testing in Identifying Toxic SubstancesMaria LabroNo ratings yet
- Assessment Form 6 (Quantitative Approach)Document16 pagesAssessment Form 6 (Quantitative Approach)Raymond RamirezNo ratings yet
- Jurnal Sentence Completion Test (SCT)Document15 pagesJurnal Sentence Completion Test (SCT)Sarah GracyntiaNo ratings yet
- Guidelines For Students Module 6 and 7Document4 pagesGuidelines For Students Module 6 and 7JasellePanteNo ratings yet
- Health Assessment Activity 1/camad, Johainah B BSN1BDocument2 pagesHealth Assessment Activity 1/camad, Johainah B BSN1Bjohainah camad100% (1)
- Introduction To PhlebotomyDocument8 pagesIntroduction To PhlebotomyGreniyelNo ratings yet
- Nominated Representative and Signatories: Responsibilities, Qualifications and ApprovalDocument10 pagesNominated Representative and Signatories: Responsibilities, Qualifications and ApprovalGerrit van der WaltNo ratings yet
- BioControl Products 1 2 TestDocument1 pageBioControl Products 1 2 Testapi-3697331No ratings yet
- 5-PPT For HR Used in MLSDocument11 pages5-PPT For HR Used in MLSNeelu MishraNo ratings yet
- NBP Planner For NEET-2023 - For Branches (Weekdays) - Version 2.0Document2 pagesNBP Planner For NEET-2023 - For Branches (Weekdays) - Version 2.0Kshitij SharmaNo ratings yet
- Alternatives To Animal TestingDocument5 pagesAlternatives To Animal TestingNiera DelossantisNo ratings yet
- Journal of Nutrition College, Volume 2, Nomor 3, Tahun 2013, Halaman 339-349Document11 pagesJournal of Nutrition College, Volume 2, Nomor 3, Tahun 2013, Halaman 339-349Ejat PhallyNo ratings yet
- Lab consumables and equipment listDocument4 pagesLab consumables and equipment listRx “Pattabi” PharmacistNo ratings yet
- Supplier Assessment Approval and Qualification Listed and Complementary MedicinesDocument14 pagesSupplier Assessment Approval and Qualification Listed and Complementary MedicinesPurwaning Nugroho WNo ratings yet
- 抗壓性格量表之編製及信度、效度的建立Document30 pages抗壓性格量表之編製及信度、效度的建立Sam Chi ChiNo ratings yet
- Amson Vaccines & Pharma (PVT) LTDDocument12 pagesAmson Vaccines & Pharma (PVT) LTDHassan Ahmed KhanNo ratings yet
- 2022 Virtual Eprs ForumDocument122 pages2022 Virtual Eprs ForumAljhan LingaNo ratings yet
- Gabriela Silang General Hospital: Republic of The PhilippinesDocument2 pagesGabriela Silang General Hospital: Republic of The PhilippinesJeremiash Noblesala Dela CruzNo ratings yet
- I S Eniso12944-9-2018Document17 pagesI S Eniso12944-9-2018que vinhNo ratings yet
- ASTM E300-03 Standard Practice For Sampling Industrial ChemicalsDocument27 pagesASTM E300-03 Standard Practice For Sampling Industrial ChemicalsQuimica Grupo 3No ratings yet
- Sentinel Aldolase OUS For The Atellica CH Rev. 5.0Document2 pagesSentinel Aldolase OUS For The Atellica CH Rev. 5.0Mauricio IbarbeNo ratings yet
- TASK 8 - Stanbio Glucose Oxidase MethodDocument2 pagesTASK 8 - Stanbio Glucose Oxidase MethodJ Pao Bayro - LacanilaoNo ratings yet
- B55by41zv3qeas2j2rh30cxvDocument2 pagesB55by41zv3qeas2j2rh30cxvHarsh SinghalNo ratings yet
- Good Microbiological Laboratory Practices Value and Recent Changes To A Guidance of Quality Laboratory PracticesDocument26 pagesGood Microbiological Laboratory Practices Value and Recent Changes To A Guidance of Quality Laboratory PracticessanthoshkurvaNo ratings yet
- Hypothesis Testing Session OutlineDocument12 pagesHypothesis Testing Session OutlineSuhuyini GaribugliNo ratings yet