Professional Documents
Culture Documents
制药工程论坛 关于手动积分指南
Uploaded by
66492334Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
制药工程论坛 关于手动积分指南
Uploaded by
66492334Copyright:
Available Formats
FDA
FDA
CFDA FDA 2016
GMP
Reintegration
FDA 2001 Bioanalytical Method Validation 2013
2013 III.C
SOP
VII.D
EMA 2011 Guideline on bioanalytical method validation 5.5
SOP SOP
https://mp.weixin.qq.com/s/kmxNftRFmqT9Ro0MCa--1w 2018/10/31 上午10B06
第 1 ⻚页(共 15 ⻚页)
1
2
3
4
5 /
QC QC QC
QC
3
QC QC
CAPA
QP
https://mp.weixin.qq.com/s/kmxNftRFmqT9Ro0MCa--1w 2018/10/31 上午10B06
第 2 ⻚页(共 15 ⻚页)
Waters Angilent
+ QA
What is Manual Integration?
So far we have discussed the regulatory issues around peak integration the
main integration parameters and the three rules of integration so that you
have a fighting chance for automatically integrating your peaks and there is
no need to intercede manually. However we now need to turn our attention
to manual integration. As we can see from the regulatory citations and the
bioanalytical guidance documents what is needed is an SOP to control
manage and authorize integration — both automatic and manual.
/ SOP
https://mp.weixin.qq.com/s/kmxNftRFmqT9Ro0MCa--1w 2018/10/31 上午10B06
第 3 ⻚页(共 15 ⻚页)
In an ideal world there would be a definition of manual integration. However
there is not a definition available for manual integration that I could find.
Neither in Dyson nor on any regulatory website. This was the point made by
Hill et al. 7 who discussed the scope and terminology of manual
reintegration and they concluded that it is essential to develop a consensus
with respect to definitions. The problem they noted is that that a
consensus does not exist.
Dyson Hill et al. 7
This raises an important question: if we can t define manual integration how
can we write an SOP on integration as a whole?
SOP
Scope of an Integration SOP
SOP
In the spirit of the early pioneers this column will present a possible
approach to writing an SOP in the form of a flowchart that will consider
some but not all options for both automatic and manual integration. Of
course there is a little trepidation because one definition of pioneer is
finding new and exotic ways to die ! However I hope that this column and
my views stimulate a debate about what constitutes manual integration and
we can get a definition agreed upon.
SOP
https://mp.weixin.qq.com/s/kmxNftRFmqT9Ro0MCa--1w 2018/10/31 上午10B06
第 4 ⻚页(共 15 ⻚页)
In this discussion we need to balance sound science with regulatory
compliance. Therefore let me pose a question — which is worse: not
reintegrating when you know the results are wrong or accepting wrong
results when you can see an unknown peak in the sample chromatograms?
You see the dilemma? We know the outcomes of both situations are
undesirable but as a result of inflexible procedures or fear of the
regulations sound scientific judgement cannot be exercised.
First let us consider a no manual integration allowed option. Personally I
think that this is an untenable situation particularly if a regulated laboratory
is using recently developed methods analyzing impurities where the limits
of quantification or detection are close to baseline noise or investigating
stability of products. In these situations manual reintegration is
scientifically sound and defendable provided that it is covered in the
integration SOP.
SOP
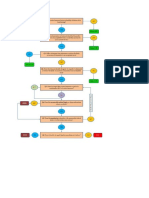
A suggested flowchart for consideration of integration is shown in Figure 3.
It begins with the completion of the chromatographic run and the automatic
integration of peaks by the original processing method. The resulting
chromatograms are reviewed by the chromatographer to see if retention
times and peak shapes are as expected the peak s have been correctly
identified baseline placement is as required by the analytical procedure
https://mp.weixin.qq.com/s/kmxNftRFmqT9Ro0MCa--1w 2018/10/31 上午10B06
第 5 ⻚页(共 15 ⻚页)
and the sample is integrated consistently with the standard. There may be
other criteria that an individual laboratory wishes to apply. We now come to
a decision point: is the run acceptable? If yes the individual results and the
reportable value are calculated and all is well. All data at this point have
been calculated automatically by the CDS the chromatographer is merely
confirming what the software has done conforms to pre-defined
expectations.
3
CDS
However if the integration is not acceptable we move to a second decision
point: is manual integration whatever that term may cover permitted for
this analytical procedure? If not the next stage is a laboratory investigation.
What methods could we consider for inclusion for no manual integration?
Perhaps measurement of active pharmaceutical ingredients APIs or
registered methods for finished product for the active ingredient? If these
peaks cannot be integrated correctly are you out of control? We will
consider if this is tenable when we have finished discussing the flowchart.
APIs active pharmaceutical
ingredients
In my view manual integration must be specifically prohibited in the
following circumstances:
https://mp.weixin.qq.com/s/kmxNftRFmqT9Ro0MCa--1w 2018/10/31 上午10B06
第 6 ⻚页(共 15 ⻚页)
Symmetrical peaks that have acceptable baseline to baseline fitting
following automatic integration.
Enhancing or shaving peak areas to meet SST acceptance criteria or
allowing a run to meet the test specification.
SST
Now we come to what constitutes manual integration . Figure 3 presents
three options that I have selected for discussion your laboratory SOP may
have more areas depending on the work performed. The outcome you
require is consistent and appropriate manual integration that is scientifically
defensible. Therefore you need to avoid situations where you have
inconsistent or inappropriate integration.
3 3
SOP
Manual Intervention Versus Manual Integration
VS
In Figure 3 options 1 and 2 are shown as manual intervention and option 3
as manual integration. Let me clarify my reasoning.
3 1 2 3
Option 1: Peaks have slipped out of a window and they are not correctly
identified. The automatic integration is acceptable and all that is
required is to change the peak windows in the integration method and
reprocess. Peak areas are not changed by this approach.
https://mp.weixin.qq.com/s/kmxNftRFmqT9Ro0MCa--1w 2018/10/31 上午10B06
第 7 ⻚页(共 15 ⻚页)
1
Option 2: Parameters in the integration or processing method need to
be adjusted and then applied to all injections in the run. An example
could be change of the peak threshold or minimum area to reduce the
impact of baseline noise. Peak areas may or may not be changed under
this option but there is no manual placement of the baselines by an
analyst.
2
Option 3: Manual placement of baselines by the chromatographer is
required because of a late running peak or noise if undertaking an
impurity analysis. Peaks areas will be changed by the reintegration.
3 late running peak
https://mp.weixin.qq.com/s/kmxNftRFmqT9Ro0MCa--1w 2018/10/31 上午10B06
第 8 ⻚页(共 15 ⻚页)
Figure 3: A suggested flow chart for manual intervention and manual
integration.
Options 1 and 2 are manual intervention but the baseline placement is
performed by the CDS and is not altered by a chromatographer. These are
the preferred options and easier to justify scientifically. Option 3 is where
everything else has failed and a chromatographer goes through individual
chromatograms and repositions the baselines where appropriate. This latter
point is important it is an exercise of scientific judgement that needs to be
backed up by the procedures within the integration SOP.
1 2 CDS
3
SOP
It s All Plain Sailing Now?
https://mp.weixin.qq.com/s/kmxNftRFmqT9Ro0MCa--1w 2018/10/31 上午10B06
第 9 ⻚页(共 15 ⻚页)
Let us return to the scenario we discussed earlier at the top of the flowchart
in Figure 3. If the automatic integration has failed because the peaks have
slipped out of the retention windows option 1 in the manual intervention
section do you want to trigger a laboratory investigation? Especially when
the peak windows are readjusted and after reintegration the run now
passes. The peaks are now correctly labelled and the peak areas are
unchanged after the manual intervention. Although the FDA classifies this
situation under manual integration there is no change to the actual
measurement of the peaks of interest. Should this situation be classified as
manual integration? In my opinion no – this is a manual intervention but not
reintegration. This is not intended to be word play but a means of trying to
define exactly what the regulators want in light of zero guidance and
multiple citations on the subject. Furthermore because there is no agreed
definition of the term we have a problem – would this be permitted? Would
you take the Clint Eastwood approach to risk management by feeling lucky
or could you justify that this is an acceptable practice?
3
1
FDA
-
- Clint Eastwood
Ideally the SST standards and QC samples should be integrated the same
way consistently throughout the run however this may not be the case
particularly near limits of quantification or detection. Let us look at a
different situation that can arise when operational efficiency is considered
and different material types are analyzed in a single run. Although the
https://mp.weixin.qq.com/s/kmxNftRFmqT9Ro0MCa--1w 2018/10/31 上午10B06
第 10 ⻚页(共 15 ⻚页)
method is validated for these different sample types there can be
differences in the chromatography that require different integration
parameters. So how can you apply the same integration parameters across
the run? The classic example is mixing APIs and stability samples where the
methods have different aims for example determination of purity versus
degradation. Perhaps the case of data integrity should win over operational
efficiency and it would be better to separate different types of analytical
procedure?
SST QC
APIs
VS
Regardless of the content of the final SOP chromatographers must be
trained to perform manual integration using a scientifically sound
justifiable and transparent process. We are looking at consistency of an
integration technique among all trainees to ensure a consistent approach
which can be provided via a CDS by copying methods and chromatograms
to a training project or directory and allowing chromatographers to integrate
the same files. One outcome of the training is that trained
chromatographers will think about where they place a baseline when
manually integrating a peak so that it is scientifically justified and complies
with the laboratory procedure.
SOP
CDS
https://mp.weixin.qq.com/s/kmxNftRFmqT9Ro0MCa--1w 2018/10/31 上午10B06
第 11 ⻚页(共 15 ⻚页)
Another outcome of the training is that all trainees must understand that
unauthorized manual integration outside of the scope of the SOP is
considered as data falsification. The more times that a run is reprocessed
either via manual intervention or manual integration the more quality
assurance and regulatory scrutiny it will attract that is the larger the size
of the red rag. Unfortunately large red rags are not very effective at
stopping regulatory bulls.
SOP
As a final comment chromatographic integration should be included in the
data integrity self-inspections to ensure that the training and procedure are
being complied with in operational use.
Summary
We have discussed manual integration in the context of a regulated
laboratory and have found a number of problems. Regulations and guidance
are lacking in GMP and the only guidance on the subject is for regulated
bioanalytical laboratories which can result in an overly bureaucratic
approach. However we lack an agreed definition of what constitutes manual
integration. Not withstanding this minor problem a suggested workflow for
determining how manual intervention and manual integration can be
combined in an overarching SOP on chromatographic integration to meet
regulatory concerns. I hope this will stimulate a debate to define what
constitutes manual integration and that regulatory agencies will provide
https://mp.weixin.qq.com/s/kmxNftRFmqT9Ro0MCa--1w 2018/10/31 上午10B06
第 12 ⻚页(共 15 ⻚页)
guidance on the subject — eventually.
GMP
SOP
1.
1
2
3
4
5 /
2
2014 FDA 483
Noprocedure exists
describing how to perform manual integration 2007 8 FDA
Leiner Health Products
manipulation of data
FDA
FDA
https://mp.weixin.qq.com/s/kmxNftRFmqT9Ro0MCa--1w 2018/10/31 上午10B06
第 13 ⻚页(共 15 ⻚页)
1
2 API
Reintegration
FDA 2001 Bioanalytical Method Validation 2013
2013 III.C
SOP
VII.D
EMA 2011 Guideline on bioanalytical methodvalidation 5.5
SOP
SOP
5
Hill
SOP
2015 11 ISPE Bob McDowall Mark
Newton Manual Intervention Manual
Integration
1 2
https://mp.weixin.qq.com/s/kmxNftRFmqT9Ro0MCa--1w 2018/10/31 上午10B06
第 14 ⻚页(共 15 ⻚页)
1
FDA
Bob
2 IPEM
SOP
https://mp.weixin.qq.com/s/kmxNftRFmqT9Ro0MCa--1w 2018/10/31 上午10B06
第 15 ⻚页(共 15 ⻚页)
You might also like
- Critical Chain Project Management: A Concept Used By The Great Military and Aerospace Companies of The World.From EverandCritical Chain Project Management: A Concept Used By The Great Military and Aerospace Companies of The World.No ratings yet
- Creating a One-Piece Flow and Production Cell: Just-in-time Production with Toyota’s Single Piece FlowFrom EverandCreating a One-Piece Flow and Production Cell: Just-in-time Production with Toyota’s Single Piece FlowRating: 4 out of 5 stars4/5 (1)
- 33-37 Statistical Process ControlDocument4 pages33-37 Statistical Process Controlyusufraf_217797493No ratings yet
- 4 Key Considerations When Engaging A New GMP Contract Service ProviderDocument5 pages4 Key Considerations When Engaging A New GMP Contract Service ProviderMina Maher MikhailNo ratings yet
- Poka Yoke PDFDocument14 pagesPoka Yoke PDFDarlyn RodriguezNo ratings yet
- Poka Yoke UsageDocument8 pagesPoka Yoke Usagejocelyn perez villarrealNo ratings yet
- Cuello de Botella Flow ShopDocument10 pagesCuello de Botella Flow ShopEdiSon JimenezNo ratings yet
- Honeywell Webinar QA Is Your PID Controller Performance Up To ParDocument4 pagesHoneywell Webinar QA Is Your PID Controller Performance Up To PartsipornNo ratings yet
- PHADocument19 pagesPHAJacekNo ratings yet
- Model Predictive Control - Camacho BourdounsDocument294 pagesModel Predictive Control - Camacho BourdounsJayram Valluru100% (1)
- 8D Bearing ExampleDocument10 pages8D Bearing Examplemech054No ratings yet
- Achieving 6 Sigma Quality in Medical Device Manufacturing by Use of DoE and SPCDocument9 pagesAchieving 6 Sigma Quality in Medical Device Manufacturing by Use of DoE and SPCispam28No ratings yet
- HTTP WWW - Pharmamanufacturing.com Articles 2007 127Document7 pagesHTTP WWW - Pharmamanufacturing.com Articles 2007 127Pradeep MishraNo ratings yet
- APQP RevisitedDocument5 pagesAPQP RevisitedRita LepesiwthNo ratings yet
- PID Auto TuningDocument84 pagesPID Auto TuningtrshaaaNo ratings yet
- Solving Supply Chain Problems ProactivelyDocument10 pagesSolving Supply Chain Problems ProactivelyGuru CharanNo ratings yet
- Project Report On Taguchi Method 9tqm Project) by Sudeshna DashDocument17 pagesProject Report On Taguchi Method 9tqm Project) by Sudeshna DashODISHA FOOD ZONE & LIFE STYLENo ratings yet
- Strategic Aspects of Retrofit: Or: What Do I Need To Think About?Document4 pagesStrategic Aspects of Retrofit: Or: What Do I Need To Think About?pankajssharmaNo ratings yet
- GIEFD U5 Diego Ortiz ARDocument8 pagesGIEFD U5 Diego Ortiz ARDiegoNo ratings yet
- Control Theory - Control Loop Tuning - Mipac IntranetDocument13 pagesControl Theory - Control Loop Tuning - Mipac IntranetKolyo RoydevNo ratings yet
- Automation in ManufacturingDocument9 pagesAutomation in ManufacturingArwind khannaNo ratings yet
- Manual 3 - QMDocument8 pagesManual 3 - QMCleber RochaNo ratings yet
- DL Prinzipienpapier BDAI enDocument16 pagesDL Prinzipienpapier BDAI encNo ratings yet
- FI Concepts: Way To ImproveDocument8 pagesFI Concepts: Way To ImproveSmith F. JohnNo ratings yet
- Manufacturing PlanningDocument13 pagesManufacturing Planningthanhvan34No ratings yet
- ISO 9001 & ISO/TS 16949: Management'S Sins Relative To SPC: Smithers Quality Assessments, IncDocument11 pagesISO 9001 & ISO/TS 16949: Management'S Sins Relative To SPC: Smithers Quality Assessments, IncPedroNo ratings yet
- Control Charts in Statistical Quality ControlDocument19 pagesControl Charts in Statistical Quality Controlanon_785745540No ratings yet
- SOP Corrective and Preventive Action (CAPA)Document3 pagesSOP Corrective and Preventive Action (CAPA)Praphul PaswanNo ratings yet
- Poka Yoke - A Misunderstood Concept - ExcellentDocument2 pagesPoka Yoke - A Misunderstood Concept - ExcellentSelvaraj BalasundramNo ratings yet
- Case Study On Poka-Yokes SresthDocument6 pagesCase Study On Poka-Yokes SresthSresth VermaNo ratings yet
- Detecting GMP FailuresDocument7 pagesDetecting GMP Failuresbsetiawan0% (1)
- Inprocess Inspection - Quantity Confirmation During Results Recording - SAP BlogsDocument8 pagesInprocess Inspection - Quantity Confirmation During Results Recording - SAP Blogsdokk100% (2)
- 26 Process Safety Management: Quiz 1 (20 Points)Document7 pages26 Process Safety Management: Quiz 1 (20 Points)Rishi TandonNo ratings yet
- Measurement Quality Objectives and Validation TemplatesDocument48 pagesMeasurement Quality Objectives and Validation TemplatesksbbsNo ratings yet
- 32.815-610 Online Charging System (OCS) Architecture StudyDocument21 pages32.815-610 Online Charging System (OCS) Architecture Studysamir_ttNo ratings yet
- Statistical Process Control A3Document14 pagesStatistical Process Control A3Sahil Sandooja100% (1)
- Updates On CPA Board ExaminationDocument6 pagesUpdates On CPA Board ExaminationMark Domingo MendozaNo ratings yet
- Available To Promise (ATP) and The Scope of Check - SAP BlogsDocument12 pagesAvailable To Promise (ATP) and The Scope of Check - SAP BlogsPrasad Shanware100% (1)
- Automation in Manufacturing: February 1997Document9 pagesAutomation in Manufacturing: February 1997anujNo ratings yet
- Application of Statistical Process Control (SPC) in ManufacturingDocument4 pagesApplication of Statistical Process Control (SPC) in ManufacturingWinny AndaliaNo ratings yet
- Planning and Implementing POLCA A Card Based Control System For High Variety or Custom Engineered ProductsDocument16 pagesPlanning and Implementing POLCA A Card Based Control System For High Variety or Custom Engineered ProductsŘüđĥirSiddhamNo ratings yet
- ARG FMA-EMPDevelopment WhitePaperDocument25 pagesARG FMA-EMPDevelopment WhitePaperJose AlfredoNo ratings yet
- Trucking ThesisDocument6 pagesTrucking ThesisScott Faria100% (2)
- Value Engineering ProposalDocument3 pagesValue Engineering ProposalSMAHMADINo ratings yet
- Toyota Case StudyDocument10 pagesToyota Case StudyYousra MariumNo ratings yet
- Realities Of: Supply Chains Must Scrutinize The UnknownDocument6 pagesRealities Of: Supply Chains Must Scrutinize The Unknownanand_sapNo ratings yet
- Integration of Value Stream Mapping With DMAIC For Concurrent Lean-Kaizen: A Case Study On An Air-Conditioner Assembly LineDocument17 pagesIntegration of Value Stream Mapping With DMAIC For Concurrent Lean-Kaizen: A Case Study On An Air-Conditioner Assembly LineJorge Durán MartínezNo ratings yet
- ISO 22000 2018 Resource CenterDocument11 pagesISO 22000 2018 Resource Centerjoyrjael100% (1)
- Project Final Report: Innovative Proactive Quality Control System For In-Process Multi-Stage Defect Reduction"Document38 pagesProject Final Report: Innovative Proactive Quality Control System For In-Process Multi-Stage Defect Reduction"Moazzam AliNo ratings yet
- Bureau Veritas India (Iatf 16949) (Page 4 of 6) Optional-1Document5 pagesBureau Veritas India (Iatf 16949) (Page 4 of 6) Optional-1ashish mehtaNo ratings yet
- Six Sigma Interview Questions and Answers UpdatedDocument14 pagesSix Sigma Interview Questions and Answers UpdatedVyas ZNo ratings yet
- QTG PerspectivesDocument12 pagesQTG PerspectiveszudNo ratings yet
- Annual Product Review Process For Pharma Industry Part1Document6 pagesAnnual Product Review Process For Pharma Industry Part1niteshnagpalNo ratings yet
- Automation in Manufacturing PDFDocument9 pagesAutomation in Manufacturing PDFParesh BobdeNo ratings yet
- Keep Advanced Control Systems OnlineDocument29 pagesKeep Advanced Control Systems OnlineBisto MasiloNo ratings yet
- Breakthrough Improvement For Your Inspection ProcessDocument11 pagesBreakthrough Improvement For Your Inspection ProcessabanzabalNo ratings yet
- BRC026 - Understanding Root Cause AnalysisDocument21 pagesBRC026 - Understanding Root Cause AnalysisLammie Sing Yew Lam100% (2)
- BRC WorkbookDocument32 pagesBRC WorkbookSâu Mập ÚNo ratings yet
- Smude Mba Sem2 All AssignmentsDocument168 pagesSmude Mba Sem2 All AssignmentsAshok BabuNo ratings yet
- Earth & Life Science: Quarter 2-Week 1Document7 pagesEarth & Life Science: Quarter 2-Week 1katy PNo ratings yet
- IRPrestige21 Users System GuideDocument78 pagesIRPrestige21 Users System GuideSilezio FerreiraNo ratings yet
- Setting Goals New Year 48412 Article - Write - Prompt - and - QuizDocument5 pagesSetting Goals New Year 48412 Article - Write - Prompt - and - QuizanaNo ratings yet
- Lecture 01 ADVANCED ANIMATION AS3Document11 pagesLecture 01 ADVANCED ANIMATION AS3Febb RoseNo ratings yet
- 5 Fit Tolerances-Problem SolvingDocument10 pages5 Fit Tolerances-Problem SolvingSHIVANANDA DALEINo ratings yet
- Eng Pcdmis 2022.1 CMM ManualDocument453 pagesEng Pcdmis 2022.1 CMM ManualRahulNo ratings yet
- A Study On Five International Scientists: Micro Project ReportDocument18 pagesA Study On Five International Scientists: Micro Project ReportAishwarya MohanNo ratings yet
- Institute of Southern Punjab Multan: Syed Zohair Quain Haider Lecturer ISP MultanDocument47 pagesInstitute of Southern Punjab Multan: Syed Zohair Quain Haider Lecturer ISP MultanCh MaaanNo ratings yet
- Building Social Networks and Strategies For SuccessDocument32 pagesBuilding Social Networks and Strategies For SuccessGita CindyNo ratings yet
- Final Verbal Agression ResearchDocument26 pagesFinal Verbal Agression ResearchLucañas PhilipNo ratings yet
- Course Outline: Yllana Bay View CollegeDocument10 pagesCourse Outline: Yllana Bay View CollegeCriseljosa LacapagNo ratings yet
- ISO 14031 Environmental Performance EvaluationDocument65 pagesISO 14031 Environmental Performance EvaluationSanjayaNo ratings yet
- Quantum: PhysicsDocument11 pagesQuantum: PhysicsVinodNo ratings yet
- Abraham Tilahun GSE 6950 15Document13 pagesAbraham Tilahun GSE 6950 15Abraham tilahun100% (1)
- III. Performance Task (Photo Analysis)Document14 pagesIII. Performance Task (Photo Analysis)Zayn MendesNo ratings yet
- Mark Scheme (Results) : Summer 2017Document22 pagesMark Scheme (Results) : Summer 2017Khalid AhmedNo ratings yet
- Aliquat-336 As A Novel Collector For Quartz FlotationDocument8 pagesAliquat-336 As A Novel Collector For Quartz FlotationMaicol PérezNo ratings yet
- Statistics For Business and EconomicsDocument19 pagesStatistics For Business and EconomicsSai MeenaNo ratings yet
- ASTM - E1137 - مشخصات دماسنجهای مقاومتی صنعتیDocument7 pagesASTM - E1137 - مشخصات دماسنجهای مقاومتی صنعتیhosein bagheriNo ratings yet
- CHM556 - Experiment 1 - Isolation of Caffeine From A Tea BagDocument8 pagesCHM556 - Experiment 1 - Isolation of Caffeine From A Tea Bagnabilahdaud93% (45)
- Time Schedule of Subjects International Class Program (Icp) Fmipa Unm Biology Department, Odd Semester, Academic Year 2010/2011Document2 pagesTime Schedule of Subjects International Class Program (Icp) Fmipa Unm Biology Department, Odd Semester, Academic Year 2010/2011El MuertoNo ratings yet
- Thermodynamics For Engineers 1st Edition Kroos Solutions ManualDocument25 pagesThermodynamics For Engineers 1st Edition Kroos Solutions ManualRhondaFisherjity100% (55)
- DMGTDocument139 pagesDMGTPatil TanishkNo ratings yet
- Year Mark: 20% Tool: Discussions Forum: Assignment 01: Whenever We Use Language, We Reflect Who We AreDocument3 pagesYear Mark: 20% Tool: Discussions Forum: Assignment 01: Whenever We Use Language, We Reflect Who We AreTheo MolotoNo ratings yet
- Internal Conflict EssayDocument8 pagesInternal Conflict EssayafabfoilzNo ratings yet
- PAPER (14) - Puucho PDFDocument21 pagesPAPER (14) - Puucho PDFethan tylerNo ratings yet
- 2 - Basic Theories of Gender PsychologyDocument20 pages2 - Basic Theories of Gender Psychologyergün ErgünNo ratings yet
- Community Health Nursing BookDocument96 pagesCommunity Health Nursing BookSamantha Renei SanchezNo ratings yet
- Grammar: Frequency AdverbsDocument2 pagesGrammar: Frequency AdverbsRedamanNo ratings yet
- 28-11-21 - JR - Iit - Star Co-Sc (Model-A) - Jee Adv - 2015 (P-I) - Wat-29 - Key & SolDocument8 pages28-11-21 - JR - Iit - Star Co-Sc (Model-A) - Jee Adv - 2015 (P-I) - Wat-29 - Key & SolasdfNo ratings yet