Professional Documents
Culture Documents
handa2018
Uploaded by
rocio enriquezCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
handa2018
Uploaded by
rocio enriquezCopyright:
Available Formats
Pediatrics International (2019) 61, 122–139 doi: 10.1111/ped.
13755
Review Article
Pediatric oncologic emergencies: Clinical and imaging review for
pediatricians
Atsuhiko Handa,1,2 Taiki Nozaki,1 Akari Makidono,1,3 Tetsuhiko Okabe,1,4 Yuka Morita,1,5 Kazutoshi Fujita,3,6
Masaki Matsusako, Tatsuo Kono, Yasuyuki Kurihara, Daisuke Hasegawa, Tadashi Kumamoto, Chitose Ogawa,7,8
1 3 1 7 7,8
Yuki Yuza9 and Atsushi Manabe7
Departments of 1Radiology and 7Pediatrics, St Luke’s International Hospital, Departments of 3Diagnostic Radiology and
9
Hematology and Oncology, Tokyo Metropolitan Children’s Medical Center, 8Department of Pediatric Oncology, National
Cancer Center Hospital, Tokyo, 4Department of Radiology, Yokohama City University School of Medicine, 6Department of
Radiology, Kanagawa Children’s Medical Center, Yokohama, 5Department of Radiology, University of the Ryukyus
Hospital, Okinawa, Japan and 2Department of Radiology, University of Iowa Hospitals and Clinics, Iowa City, Iowa, USA
Abstract Children with cancer are at increased risk of life-threatening emergencies, either from the cancer itself or related to
the cancer treatment. These conditions need to be assessed and treated as early as possible to minimize morbidity
and mortality. Cardiothoracic emergencies encompass a variety of pathologies, including pericardial effusion and
cardiac tamponade, massive hemoptysis, superior vena cava syndrome, pulmonary embolism, and pneumonia.
Abdominal emergencies include bowel obstruction, intussusception, perforation, tumor rupture, intestinal graft-
versus-host disease, acute pancreatitis, neutropenic colitis, and obstructive uropathy. Radiology plays a vital role in
the diagnosis of these emergencies. We here review the clinical features and imaging in pediatric patients with
oncologic emergencies, including a review of recently published studies. Key radiological images are presented to
highlight the radiological approach to diagnosis. Pediatricians, pediatric surgeons, and pediatric radiologists need to
work together to arrive at the correct diagnosis and to ensure prompt and appropriate treatment strategies.
Key words computed tomography, magnetic resonance, pediatric oncologic emergency, radiograph, ultrasound.
Children with cancer are at increased risk of life-threatening leukemia and lymphoma.3–5 Cardiac hemangioma and pericar-
emergencies, either from the cancer itself or related to the dial teratoma may cause neonatal pericardial effusion.6 The
cancer treatment.1 These conditions need to be assessed and classical findings of cardiac tamponade, known as Beck’s
treated as early as possible to minimize morbidity and mortal- triad, consist of hypotension, increased jugular venous pres-
ity. Radiological imaging plays a vital role in the diagnosis. sure, and muffled heart sounds. Symptoms and signs such as
We here review the clinical features and imaging in pediatric cough, chest pain, dyspnea, tachycardia, and pulsus paradoxus
patients with oncologic emergencies, including a review of may also be present. The rate of accumulation contributes
recently published studies. more significantly to the development of cardiac tamponade
than the ultimate volume or composition of the pericardial
fluid.7
Cardiothoracic oncologic emergencies
Chest radiograph shows a globular-shaped enlarged cardiome-
diastinal silhouette, classically called a “water bottle” shape
Pericardial effusion and cardiac tamponade
(Fig. 1a).8 Echocardiography shows a sonolucent area between
Pericardial effusions are excessive fluid in the pericardial the heart and pericardium, and may also show thickening of the
space.2 They can result in cardiac tamponade if the fluid com- pericardium suggestive of pericarditis or pericardial tumor
presses the heart to the extent that venous return and cardiac (Fig. 1b). Computed tomography (CT) demonstrates compres-
output are compromised. Pericardial effusion may be caused sion of the cardiac chambers by effusion and may also show
by tumor, opportunistic infection attributable to the patient’s nodular lesions, thickening of the pericardium, or hemorrhage.
immunocompromised state, or fibrosis from previous radia- Other CT findings in patients with tamponade include enlarge-
tion.3 The most common tumors to invade the pericardium are ment of the superior vena cava (SVC) or inferior vena cava
(IVC), periportal edema, reflux of contrast material into the IVC
Correspondence: Taiki Nozaki, MD PhD, Department of Radiol- or azygos vein, and enlargement of hepatic and renal veins.9 CT
ogy, St Luke’s International Hospital, 9-1 Akashi-cho, Chuo-ku, is superior to echocardiography in characterizing pericardial effu-
Tokyo 104-8560, Japan. Email: nojyakki@gmail.com
sion and pericardial mass because it provides a larger field of
Received 15 March 2018; revised 10 November 2018; accepted
13 December 2018. view and better visualization of anatomical details.10 Magnetic
© 2018 Japan Pediatric Society
Pediatric oncologic emergencies 123
a pulmonary aspergillosis12 or diffuse alveolar hemorrhage.13
Invasive pulmonary aspergillosis typically occurs in immuno-
compromised patients, especially those with prolonged
chemotherapy-induced neutropenia. Imaging in patients with
invasive pulmonary aspergillosis will be discussed later in the
Pneumonia section. Diffuse alveolar hemorrhage, a rare but
severe non-infectious complication of hematopoietic cell trans-
plantation (HCT), presents with respiratory failure and hemop-
tysis around the time of white blood cell engraftment,13 and is
associated with a high mortality rate.14 A recent study on
autopsies of children with cancer found a high incidence of
pulmonary hemorrhage (40%), this being the most frequently
missed clinical diagnosis.15 The absence of hemoptysis does
not exclude a diagnosis of diffuse alveolar hemorrhage in
adults;16 this may also be true for children.
Chest radiograph in patients with diffuse alveolar hemor-
rhage may show air space opacification or ground-glass opac-
ity predominantly in the perihilar region (Fig. 2a). Classically,
lesions are bilateral and symmetric, but they can also be
asymmetric or unilateral.11 CT shows patchy or diffuse
ground-glass or air space opacities (Fig. 2b). The differential
diagnosis of these CT findings includes acute respiratory dis-
tress syndrome/acute lung injury (diffuse alveolar damage),
b
cardiogenic pulmonary edema, and pneumonia. In the acute
phase, diffuse alveolar damage manifests as interlobular septal
thickening and traction bronchiectasis and tends to have a geo-
graphic distribution.17 Cardiogenic pulmonary edema mani-
fests as cardiomegaly, engorged pulmonary vessels,
interlobular septal thickening, and pleural effusion. Neuroblas-
toma may cause congestive heart failure due to cate-
cholamine-induced cardiomyopathy (Fig. 3).18 Pneumonia is
typically a focal process, as detailed in the Pneumonia section.
Follow-up imaging of diffuse alveolar hemorrhage shows reso-
lution of hemorrhage from alveolar spaces and thickening of
interlobular septa, resulting in the classical finding of a
“crazy-paving” pattern.
Mediastinal tumor causing SVC syndrome and airway
narrowing
Superior vena cava syndrome is a constellation of signs and
symptoms caused by venous congestion due to obstruction of
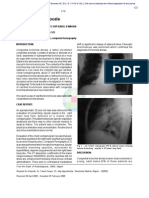
Fig. 1 Cardiac tamponade in a 4-year-old girl with myelodys- the SVC.19 Given that infants and young children have a soft
plastic syndrome. (a) Antero-posterior chest radiograph showing a and compressible airway, airway narrowing often accompanies
globular-shaped enlarged cardiac silhouette classically called a
“water bottle” shape. (b) Echocardiography showing a large SVC syndrome in this age group (“superior mediastinal syn-
sonolucent area (asterisks) between the heart and pericardium that drome”). Older children may have isolated tracheal narrowing
is compressing the cardiac chambers. or SVC syndrome. SVC syndrome can be caused by extrinsic
compression or intrinsic obstruction. The most common cause
resonance (MR) imaging can further characterize effusions,11 but of SVC syndrome is an anterior mediastinal mass, such as
it is less often used to diagnose pericardial effusion. lymphoma and leukemia (such as precursor T-cell lymphoblas-
tic lymphoma or T-cell acute lymphoblastic leukemia;
ALL).19 Neuroblastoma, germ-cell tumors, and sarcomas are
Hemoptysis
also common etiologies.19 Prolonged placement of central
Hemoptysis can occur in children with cancer.1 Mild hemopty- venous catheter puts children at risk of acquiring SVC throm-
sis is usually attributable to aspiration of epistaxis. Moderate bosis.20 Classical symptoms of SVC syndrome in adults
to severe hemoptysis can occur in patients with invasive include edema and plethora of the face, neck, and arm, as well
© 2018 Japan Pediatric Society
124 A Handa et al.
supine position may aggravate respiratory symptoms. CT may
a
be obtained in the prone position in such cases. MR venogra-
phy can provide evaluation of vascular flow without the use
of contrast material,11 but MR often requires sedation and
may not be optimal in the setting of acute respiratory compro-
mise. CT is still preferred over MR because of its speed and
ability to demonstrate pulmonary pathology.22
Diagnosis of SVC syndrome caused by mediastinal mass
should be made in a step-wise approach starting with the least
invasive and lowest risk technique possible to avoid cardiores-
piratory compromise.21,23 Peripheral blood test, pleural fluid
cytology and analysis, bone marrow aspiration, or open/needle
biopsy of accessible extra-thoracic disease lesions (e.g. lymph
nodes) should be attempted before open biopsy requiring gen-
eral anesthesia. Multiple specialists (anesthesiologists, oncolo-
gists, surgeons, pulmonologists, and radiologists) should be
involved, and the diagnostic as well as the therapeutic plan
should be fully discussed. Benefits and risks for sedation
should be carefully weighed, given that a loss of negative tho-
racic pressure caused by sedation can further narrow a thin-
walled SVC and flexible airway, which can exacerbate pre-
existing symptoms,24 or, in the worst case, may lead to car-
diopulmonary arrest. Cases of peri-anesthetic death are well
known, even in children who did not have airway symptoms.
b Steroids or radiation treatment may be beneficial even before
establishing a diagnosis to prevent airway compromise.
Image-guided needle biopsy of a mediastinal mass or enlarged
lymph node can be performed under local (or general) anes-
thesia by a skilled interventional radiologist.23
Pulmonary embolism
Pediatric patients with cancer are at high risk of pulmonary
thromboembolism (PE).25 The risk of venous thromboem-
bolism, including both deep venous thrombosis and PE, is
four–sevenfold higher in patients with cancer than in those
Fig. 2 Diffuse alveolar hemorrhage resulting from hemophago- without cancer.26 Another study found that 25% of children
cytic lymphohistiocytosis in a 12-year-old boy with myelodysplas- with venous thromboembolism have an underlying neoplasm
tic syndrome who had massive hemoptysis. (a) Antero-posterior and that cancer is a major cause of mortality in children with
chest radiograph showing bilateral patchy ground-glass opacities deep venous thrombosis.27 Surprisingly, almost 2% of routine
that predominate in the perihilar region (arrowheads). (b) Axial
thoracic CT of pediatric patients with cancer showed evidence
unenhanced computed tomography showing bilateral patchy
ground-glass opacity and consolidation that is most marked cen- of PE.25 Many factors may contribute to the development of
trally. PE in children with cancer, including the use of a central
venous catheter, disease- or treatment-related (such as
asparaginase) coagulopathy, tumor lysis, parenteral hyperali-
as venous distention of the cervical and anterior chest wall mentation, and platelet microaggregates from transfusions.28
veins.20 In pediatrics, patients more commonly present with In addition to being at risk of thromboembolism, pediatric
cough, dyspnea, orthopnea, and wheeze, which may mimic patients with cancer are at risk of tumor thrombus and embo-
upper respiratory tract infection or asthma.19 lism (Fig. 7).25
Chest radiograph may show mediastinal widening20 and Radiographic findings of PE are non-specific. Chest radio-
airway compression21 in cases of mediastinal mass (Figs 4–6). graph is commonly normal.11 Classical signs of PE such as
CT with intravenous contrast materials may demonstrate tumor oligemia (“Westermark sign”) and peripheral wedge-shaped
and its involvement of the surrounding vasculature and airway infarction (“Hampton hump”) are rarely seen. CT pulmonary
(Figs 4–6). CT can also provide tracheal cross-sectional area, angiography, currently the preferred procedure for detecting
although the correlation of symptoms with imaging degree of PE, demonstrates emboli as arterial filling defects. The lung
obstruction is poor.21 Care must be taken during CT, since the parenchymal finding of wedge-shaped peripheral consolidation
© 2018 Japan Pediatric Society
Pediatric oncologic emergencies 125
a b
Fig. 3 Catecholamine-induced cardiomyopathy associated with neuroblastoma in a 3-year-old boy. (a) Baseline chest radiograph
3 weeks prior to the referral when patient started to have fever. Retrospectively, the patient had abnormal calcification in the paraspinal
regions (arrow). (b) At the time of referral, the patient had persistent fever. Chest radiograph showing engorged pulmonary vessels with
mild cardiomegaly (arrowheads). (c) Axial contrast-enhanced computed tomography showing a large retroperitoneal tumor with punctate
calcifications (arrow).
is significantly associated with PE.29 The utility of MR (often bilateral, symmetric, diffuse ground glass and reticulon-
angiography has been demonstrated in adults, albeit with cer- odular opacities) from a focal air space lesion (often unilat-
tain limitations.30 There is no clinical evidence for the useful- eral, asymmetric, patchy air space opacification).31,32 It can
ness of MR angiography in detecting PE in children, making also show complications such as parapneumonic effusion/
the role of this procedure uncertain. Nuclear ventilation perfu- empyema or lung abscess and exclude other thoracic causes of
sion or conventional pulmonary angiography are seldom used abnormality.33 Because a specific organism can cause a vari-
in children with suspected PE. ety of imaging patterns and there is significant overlap
between imaging findings of pneumonia caused by different
organisms, the causative organism can rarely be determined
Pneumonia/pulmonary infection
on the basis of imaging alone. Sometimes more than one
Pediatric patients with cancer may be at increased risk of organism is responsible for infection in immunocompromised
pneumonia because their underlying malignancies (such as children. Nevertheless, chest radiograph and chest CT play
ALL) and/or chemotherapy may compromise their immune important roles in the diagnostic workup. Chest CT is often
systems.31 Pneumonia is the commonest location of infection necessary to evaluate the possibility of a lung infection
and is a major cause of mortality. Fever may be the only ini- because the initial chest radiograph findings in patients with
tial symptom because of impairment of the inflammatory cell fungal infection or pneumocystis pneumonia may be very sub-
response. tle or even non-existent. Indeed, evaluation of the possibility
Imaging plays a major role in several ways. It may help by of lung infection is a common reason for performing chest CT
distinguishing infection manifesting as an interstitial disease in such children.
© 2018 Japan Pediatric Society
126 A Handa et al.
b c
Fig. 4 Severe superior vena cava (SVC) and airway narrowing caused by an anterior mediastinal mass from T-cell lymphoblastic lym-
phoma in a 14-year-old girl who presented with facial swelling and dyspnea. (a) Frontal chest radiograph showing a massive right-sided
pleural effusion (asterisk). (b,c) Axial contrast-enhanced computed tomography showing an anterior mediastinal tumor compressing the
trachea (arrowheads) and SVC (arrows). Collateral veins including hemiazygos and paravertebral veins have developed due to severe
stenosis of SVC (dotted arrow). A large pleural effusion is again shown.
If chest radiograph shows a pattern of interstitial disease, who have undergone HCT and have impaired cellular immu-
viruses (including cytomegalovirus [CMV], Herpes simplex nity. Epstein–Barr virus can cause post-transplantation lym-
virus, Varicella zoster virus, Epstein–Barr virus, and respira- phoproliferative disease.31
tory syncytial virus), Pneumocystis jiroveci, mycoplasma When chest radiograph shows focal, asymmetrical air
(early phase), and Chlamydophila should be considered. CT space lesions, bacterial, mycobacterial, and fungal infections
may show ground glass opacities in a centrilobular distribu- should be considered. Chest radiograph/CT may show (i)
tion (sometimes with patchy air space opacity), mosaic perfu- lobar (or segmental, spherical) pneumonia with air bron-
sion, and/or air trapping. Typical CT findings of CMV chogram (typically caused by nosocomial bacteria, myco-
pneumonia are centrilobular or randomly distributed nodules plasma (later phase), mycobacteria, and fungi; Fig. 8); or (ii)
in addition to patchy or widespread air space consolidation bronchopneumonia with patchy nodules of soft-tissue density
and ground glass opacities. Of note, in children <2 years of in a centrilobular distribution, correlating with endobronchial
age, viral pneumonia (such as respiratory syncytial virus, rhi- spread of organisms (typically caused by Staphylococcus
novirus, parainfluenza virus, human metapneumovirus, aden- aureus, Pseudomonas aeruginosa, Escherichia coli, mycobac-
ovirus, influenza virus) is typically associated with teria, and fungi), often associated with bronchial wall thick-
hyperinflation with prominent bilateral symmetric peri- ening and airway impaction (although when the latter are
bronchial markings and patchy areas of subsegmental atelec- isolated findings, viral or atypical organisms are more
tasis.34 Viruses that commonly cause mild pneumonia in likely). Tree-in-bud appearance suggests infection with pyo-
healthy children may display greater virulence in children genic bacteria (such as Streptococcus pneumoniae,
© 2018 Japan Pediatric Society
Pediatric oncologic emergencies 127
b c
Fig. 5 Severe airway narrowing caused by a large pleuropulmonary blastoma in a 2-year-old girl. (a) Frontal chest radiograph showing
complete opacification of the left hemithorax, and tracheal deviation as well as mediastinal shift to the right. (b) Coronal and (c) axial
contrast-enhanced computed tomography showing a large mixed solid and cystic tumor occupying the left hemithorax (arrows) with
mediastinal shift to the right.
Staphylococci) or mycobacteria. Encapsulated bacteria (such Last, immunocompromised children who have undergone
as S. pneumoniae, Haemophilus influenzae) show increased HCT deserve special note because they tend to be affected
virulence in children who have undergone HCT and have sequentially by various conditions during the post-transplant
impaired humoral immunity. period.37 During the initial neutropenic phase (2–3 weeks
Relatively large nodules and cavities commonly denote after transplantation), bacterial pneumonia and fungal infec-
bacterial lung abscesses, nocardiosis, fungal/mycobacterial tion may occur. Non-infectious complications include pul-
infection, or septic embolism. Multiple, scattered, bilateral monary edema, diffuse alveolar hemorrhage, and engraftment
nodular or mass-like consolidation (without bronchial wall syndrome. Engraftment syndrome is characterized by fever,
thickening) is the typical manifestation of aspergillosis rash, pulmonary edema, weight gain, liver and renal dysfunc-
(Fig. 9), mucormycosis (Fig. 10), and candida infections, all tion, and/or encephalopathy, and occurs approximately
of which warrant special attention in neutropenic children. A 10 days after HCT.38 CT findings are similar to pulmonary
halo of ground glass opacity may be seen surrounding nodules edema but without cardiomegaly.39 During the early phase
(“halo sign”); in the setting of neutropenia; this may suggest (between 2–3 weeks and 100 days), humoral immunity is still
angioinvasive aspergillosis infection.35 A crescent-shaped area impaired and there is a significant risk of Pneumocystis or
of radiolucency in a region of nodular opacity (“air-crescent cytomegalovirus pneumonia. Idiopathic pneumonia syndrome
sign”) may also be seen, but these findings are infrequent in is a common non-infectious complication, which is a clinical
younger children.36 diagnosis made by signs and symptoms of pneumonia (such
Random patterns of small nodules may be seen with mil- as hypoxia) associated with widespread alveolar injury in the
iary tuberculosis or miliary fungal infection (including histo- absence of lower respiratory tract infection.40 Chest radio-
plasmosis and coccidiomycosis). graph/CT shows multilobar air space opacification or ground
© 2018 Japan Pediatric Society
128 A Handa et al.
b c
Fig. 6 Severe airway narrowing caused by a metastatic mediastinal lymph node due to hepatoblastoma in a 1-year-old-girl who pre-
sented with abdominal distention. (a) Frontal chest radiograph showing near complete opacification of the left hemithorax without marked
mediastinal shift. Tortuous coursing of the nasogastric tube (black arrows) suggest esophageal deviation to the right. (b,c) Axial contrast-
enhanced computed tomography (CT) of the (b) chest shows a metastatic lymph node compressing the left mainstem bronchus (white
arrow), and (c) of the abdomen shows a large heterogeneous tumor replacing the left liver with peritoneal implants over the liver capsule
(arrowheads). There also is associated peritoneal free fluids (asterisk) with increased CT density suggestive of intra-abdominal tumor
rupture.
glass opacities in the bilateral lung with basilar or dorsal pre- surrounding consolidation (atoll or reverse halo sign) may be
dominance. During the late phase (between 100 days and seen. Pulmonary veno-occlusive disease is a narrowing or
1 year), non-infectious complications such as obliterative occlusion of the pulmonary venules or small veins resulting
bronchiolitis, organizing pneumonia, and pulmonary veno- in pulmonary hypertension.45 Smooth diffuse interlobular sep-
occlusive disease may occur. Obliterative bronchiolitis (or tal thickening in the absence of left-sided heart disease is the
bronchiolitis obliterans) is an inflammatory/fibrosing process most suggestive sign on CT.46
involving the small airways that often results in progressive,
irreversible obstructive pulmonary disease.41 CT shows geo-
Abdominal oncologic emergencies
graphic areas of hyperlucency and bronchial wall thickening
with mosaic pattern of lung attenuation.42 Organizing pneu-
Intestinal obstruction and intussusception
monia (previously known as bronchiolitis obliterans organiz-
ing pneumonia, BOOP) is an alveolar inflammation that may Acute small bowel obstruction can be caused by external or
occur secondary to HCT.43 CT may show peripheral patchy internal abnormalities.11 Adhesion and stricture are common
air space infiltrates.44 Central ground glass opacification with causes of obstruction in children who have undergone
© 2018 Japan Pediatric Society
Pediatric oncologic emergencies 129
Fig. 9 Invasive aspergillosis in a 2-year-old boy with prolonged
neutropenia during chemotherapy for acute lymphoblastic leuke-
Fig. 7 Tumor thrombus in a 4-year-old boy with rhabdomyosar- mia. Axial unenhanced computed tomography at the level of the
coma. Axial contrast-enhanced computed tomography showing a lung apex showing bilateral ground glass opacity surrounding
tumor thrombus (arrow) severely narrowing the right main nodular lesions (“halo sign” [arrows]). The area of radiolucency
pulmonary artery. Bilateral lung metastases are also evident (as- surrounding a region of nodular opacity in the right apex is a
terisks). The thrombus resolved after chemotherapy. fully developed “air-crescent” (asterisk). Serum antigen testing
was positive for Aspergillus.
Fig. 8 Klebsiella pneumonia in a neutropenic 12-year-old boy
during chemotherapy for Burkitt lymphoma. Axial unenhanced Fig. 10 Mucormycosis in a 10-year-old girl with relapsed acute
computed tomography showing a focal peripheral area of air lymphoblastic leukemia. Axial unenhanced computed tomography
space opacification (asterisk) with air bronchogram (arrow) and of the chest showing an area of air space opacification in the right
bronchial wall thickening (arrowhead). Blood cultures grew Kleb- apex with surrounding areas of ground glass opacity (“halo sign”
siella pneumoniae. [arrows]). Polymerase chain reaction was positive for Mucormy-
cetes.
abdominal/pelvic surgery. Tumors that may cause bowel
obstruction include sarcoma, abdominal lymphoma, large both the location and cause, including tumor (Fig. 11b), and is
renal/adrenal tumor, ovarian and colon tumors, and presacral the modality of choice.11 CT is especially helpful in patients
teratoma.1 Narcotics and vinca alkaloids can cause non- with closed-loop obstruction and obstruction related to adhe-
obstructive adynamic ileus. Children typically present with sions, demonstrating dilated bowel loops proximally and col-
persistent bilious vomiting and abdominal pain. lapsed bowel distal to the obstruction.
Abdominal radiograph classically shows multiple dilated Intussusception can be caused by benign small bowel
loops of bowel with air–fluid levels on upright or decubitus tumors (polyps, especially those associated with Peutz–Jeghers
images and absence of stool and gas in the bowel distal to the syndrome, hemangioma/vascular malformations, neurofibroma,
obstruction (Fig. 11a).11 With adynamic ileus, there is typi- fibroma, leiomyoma, gastrointestinal (GI) stromal tumor
cally uniform dilatation of the entire small and large bowels. (GIST), lipoma, and lipoblastoma) or malignant tumors (Bur-
Although abdominal radiograph may suggest the location of kitt lymphoma and carcinoid tumor).11 Intussusception is the
obstruction, CT with intravenous contrast better demonstrates initial presentation in 17–25% patients with abdominal Burkitt
© 2018 Japan Pediatric Society
130 A Handa et al.
small bowel intussusceptions found in children on US or CT
a are transient and of no clinical significance.50 Findings that
suggest transient small bowel intussusception on US include
(i) small size without wall swelling; (ii) short segment; (iii)
preserved wall motion; and (iv) absence of the lead point.51
Findings that suggest pathological intussusception include
intussusception length >3.5 cm, and evidence of bowel
obstruction or an identifiable lead point.51,52 Repeat focused
US can document spontaneous resolution when necessary.
Gastrointestinal perforation, tumor rupture, and
hemorrhage
Gastrointestinal perforation may be caused by persistent
obstruction, gastritis/peptic ulcers, or erosion caused by tumor
infiltration. Because of its tendency to involve the full thick-
ness of the bowel wall and sensitivity to chemotherapy, post-
chemotherapy intestinal rupture can occur in patients with
Burkitt lymphoma.53 Neuroblastoma (especially in neonates),
Wilms tumor, hepatoblastoma, and ovarian tumor may rarely
present with tumor rupture.54–57 Affected children typically
present with severe abdominal pain and evidence of shock
and/or peritonitis. Ovarian tumor can also present with acute
abdomen due to torsion (Fig. 12), which is often associated
with hemorrhagic necrosis in the later phase.58
Abdominal radiograph typically shows free abdominal air
in patients with bowel perforation, and free fluids in those
b
with intraabdominal hemorrhage or tumor rupture.11 US may
show a large amount of fluid in the peritoneal cavity. CT may
show the responsible tumor, hemoperitoneum (unclotted bleed
usually has attenuation of 20–60 Hounsfield units [HU], and
clotted blood, higher attenuation [60–90 HU]), and the source
of bleeding, especially if intravenous contrast demonstrates
extravasation (Figs 6c,13). Ovarian torsion presents with ovar-
ian enlargement with or without an underlying mass, but it is
non-specific. A twisted pedicle, although not often detected on
imaging, is pathognomonic when seen.58
Intestinal graft-versus-host disease
Graft-versus-host disease (GVHD), a serious complication of
Fig. 11 Small bowel intussusception and obstruction resulting
from diffuse large B-cell lymphoma in a 5-year-old boy. (a) HCT,59 occurs when donor T lymphocytes react with the tis-
Upright abdominal radiograph showing multiple dilated loops of sues of the host, such as those in the skin, liver, and GI tract.
small bowel with air–fluid levels (white arrowheads) suggestive For GVHD to develop, (i) the graft must contain immunologi-
of small bowel obstruction. (b) Axial contrast-enhanced computed cally competent cells; (ii) the recipient must express tissue
tomography showing small bowel obstruction caused by intussus- antigens that are not present in the transplant donor; and (iii)
ception (arrow), with enlarged lymph nodes being a pathological
lead point (black arrowhead). the recipient must be incapable of mounting a response cap-
able of destroying the transplanted cells.60 The most important
risk factor for the development of GVHD is human leukocyte
lymphoma.47,48 Physicians should strongly suspect underlying antigen mismatch.61 Acute GVHD is usually diagnosed on a
pathology when older children (aged >6 years) present with clinical basis, but it is often difficult to differentiate from
intussusception.48 thrombotic microangiopathy or CMV infection clinically. It
Abdominal radiograph may show evidence of obstruction, occurs in the first 100 days after transplantation. Patients usu-
as noted in the previous section. Ultrasound (US) and CT ally present with a pruritic maculopapular rash, hepatitis (jaun-
show bowel within bowel (“target/doughnut sign”, “pseudo- dice), and GI abnormalities (crampy abdominal pain, nausea,
kidney sign” or “concentric ring sign” on US).49 Of note, most vomiting, and profuse diarrhea). Pathologically, acute GVHD
© 2018 Japan Pediatric Society
Pediatric oncologic emergencies 131
a b
Fig. 12 Left adnexal torsion in a 14-year-old girl who presented with acute abdomen. (a) Axial unenhanced computed tomography (CT)
of the pelvis showing a large mass with internal septations and increased CT density in the dependent portion (arrowhead) suggestive of
hemorrhagic component. (b) Axial contrast-enhanced CT of the pelvis showing a large tumor without solid enhancing components. The
adnexal mass also appears to lack wall enhancement suggestive of necrosis. (c) Coronal contrast-enhanced CT of the pelvis showing mild
surrounding fat stranding and free fluid in the pelvic floor (asterisk).
is characterized by selective epithelial damage in target part of the intestine, but the small intestine is characteristically
organs.61 In intestinal GVHD, destruction of intestinal crypts, most severely affected. Pneumatosis intestinalis can be seen, as
especially at the base of the crypts (which contain a high pro- detailed in the next paragraph (Fig. 15).65 Common extra-intest-
portion of stem cells), results in patchy or diffuse mucosal inal findings include engorgement of the mesenteric vessels and
ulceration. ascites. Abnormal enhancement of the gallbladder or bladder
Abdominal radiograph shows ileus with separation of bowel wall may also occur, but these findings are non-specific. The
loops, thickening of the bowel wall, and air–fluid levels.62 US differential diagnosis includes neutropenic colitis, infectious
shows bowel wall thickening (>3 mm) and dilated fluid-filled enterocolitis including pseudomembranous colitis and viral
loops of bowel. CT with intravenous contrast shows striking infection, and tumor involvement (Fig. 16).64 Neuroblastoma
mucosal enhancement that correlates histopathologically with and ganglioneuroblastoma may also cause refractory diarrhea as
mucosal destruction (Fig. 14).63 The mucosal and serosal sur- a paraneoplastic syndrome.66 Neutropenic colitis, as detailed in
faces avidly enhance, thus appearing hyperattenuating, and cre- a following section, most commonly involves the cecum and
ate a “target” sign with a hypoattenuating middle layer occasionally the ileum. Pseudomembranous colitis usually man-
representing intramural or submucosal bowel wall edema.64 ifests as a pancolitis with marked fold thickening and rarely
Bowel wall thickening and luminal narrowing may affect any involves the small bowel. The distribution of CMV colitis is
© 2018 Japan Pediatric Society
132 A Handa et al.
Fig. 13 Wilms tumor rupture in a 2-year-old girl. (a) Coronal
and (b) axial contrast-enhanced computed tomography showing a
left renal tumor with obscured anterior–inferior margin (arrow)
and massive hemorrhage (asterisks) into the retroperitoneal and
peritoneal spaces. Histopathology showed ruptured Wilms tumor.
similar to that of neutropenic colitis. Although there is some
overlap between GVHD and other conditions, the location and Fig. 14 Intestinal graft-versus-host disease (GVHD) in a 9-year-
extent of bowel involvement and presence of associated findings old girl with acute lymphoblastic leukemia who developed severe
can help narrow the differential diagnosis. Given that differenti- vomiting 50 days after hematopoietic cell transplantation. (a) Cor-
ation of intestinal GVHD from infective enteritis is critical to onal and (b) axial contrast-enhanced computed tomography show-
selecting the appropriate treatment, tissue sampling via biopsy ing dilated fluid-filled loops of bowel, diffuse bowel wall
thickening (arrowheads) and striking contrast enhancement of the
may be required. bowel wall. Upper and lower gastrointestinal endoscopy indicated
Pneumatosis intestinalis is occasionally associated with mucosal erythema, and biopsy confirmed the diagnosis of intesti-
intestinal GVHD. In a recent study, 10.1% of children who nal GVHD.
© 2018 Japan Pediatric Society
Pediatric oncologic emergencies 133
a b
Fig. 15 Extensive pneumatosis associated with intestinal graft-versus-host disease (GVHD) in an 11-year-old girl with malignant lym-
phoma who had undergone hematopoietic cell transplantation. (a) Supine abdominal radiograph showing curvilinear foci of gas outlining
the ascending and transverse colon with dilated bowel suggestive of extensive pneumatosis coli (white arrows). There is no evidence of
intra-abdominal free air. (b) Coronal contrast-enhanced computed tomography (CT) in the lung window showing extensive circumferen-
tial collections of air in the wall of the colon that runs parallel to the ascending and transverse colon (black arrows), indicating a rela-
tively benign form of intestinal GVHD. (c) Axial contrast-enhanced computed tomography again showing pneumatosis without what
would be clinically more worrisome features such as bowel wall thickening, loss of contrast enhancement, or surrounding fat stranding.
There is diffuse mild thickening of the small bowel wall (black arrowheads) attributable to GVHD. The patient recovered without
surgery.
had received allogenic HCT developed pneumatosis intesti- findings have been inconsistent, and no strong basis has been
nalis a median of 94 days after transplant; they were success- established for such genetic risk factors.71,72 Other medication
fully managed medically.67 Surprisingly, extensive associated with pancreatitis includes corticosteroids, 6-mercap-
pneumatosis indicates a relatively benign form of intestinal topurine, and trimethoprim–sulfamethoxazole.69
GVHD, whereas bowel wall thickening, free peritoneal fluid, The diagnosis of acute pancreatitis rests on clinical and lab-
and peri-intestinal soft-tissue stranding are associated with oratory findings (an elevation of amylase and lipase), and
more serious clinical manifestations.68 imaging can both help confirm the diagnosis and detect com-
plications.69,70 Depending on local practice, imaging may not
necessarily be pursued, but the relatively high incidence of
Acute pancreatitis
pseudocysts probably warrants imaging in all cases.70 US can
Acute pancreatitis can be caused by drugs such as asparaginase, be normal in mild acute pancreatitis. In moderate–severe
an agent used in the treatment of ALL and lymphoma.69,70 cases, the pancreas is enlarged and hypoechoic (Fig. 17a).11
Affected patients present with abdominal pain, nausea, and CT demonstrates focal or diffuse pancreatic enlargement,
vomiting, typically soon after starting asparaginase therapy. mildly decreased enhancement, surrounding fat inflammation,
Recent studies have tried to identify genetic risk factors for and often peripancreatic fluid collection (Fig. 17b). Pseudocyst
asparaginase-related pancreatitis in children with ALL, but the formation is the commonest complication, occurring in 18–
© 2018 Japan Pediatric Society
134 A Handa et al.
b c
Fig. 16 Langerhans cell histiocytosis in a 7-month-old girl presenting with profuse diarrhea. (a) Coronal and (b) axial contrast-enhanced
computed tomography of the abdomen and pelvis showing diffuse dilatation of the bowels, intraperitoneal free fluids (asterisks), and sple-
nomegaly suggestive of gastrointestinal involvement. (c) Contrast-enhanced fat-saturated axial T1-weighted magnetic resonance imaging
showing an enhancing mass in the hard palate (arrows).
30% of patients.69,70,73 Other complications include necrosis,
Neutropenic colitis
abscess, and hemorrhage. Plain film findings are non-specific,
but certain findings are suggestive.11 Reactive ileus of nearby Neutropenic colitis, or typhlitis, is a necrotizing colitis that
GI structures (“sentinel loops”), air–fluid levels in stomach usually involves the cecum, ascending colon, appendix, and
and duodenum, focal dilatation of the duodenal sweep, and terminal ileum, typically in neutropenic children with hemato-
dilatation of the transverse colon ending abruptly at the sple- logic malignancies.1,74 Neutropenic colitis can occur else-
nic flexure (“colon cut-off sign”), are rare but classical find- where: the transverse, descending colon, or rectum being
ings in patients with moderate–severe pancreatitis. Left pleural involved in 40% of cases.74 Most (but not all) children with
effusion may also occur. neutropenic colitis present with abdominal pain, fever,
Re-treatment with asparaginase after an episode of mild abdominal tenderness, and diarrhea.74 Typically, neutropenic
pancreatitis is associated with a higher risk of recurrent pan- colitis develops when the absolute neutrophil count declines
creatitis. The risk of the second episode of asparaginase-asso- sharply to a nadir after chemotherapy for acute leukemia.75
ciated pancreatitis, however, does not depend on the severity The postulated mechanism is that chemotherapy-related
of the first episode, and the second episode does not have a destruction of the normal mucosal architecture is followed by
greater risk of complications.70 The current consensus on deci- bacterial/fungal invasion of the bowel facilitated by decreased
sions about asparaginase re-exposure appears to be that they defenses due to neutropenia, leading to neutropenic coli-
should be based on balancing the importance of asparaginase tis.76,77
in achieving leukemia survival versus the risk of the second Ultrasound or CT typically show bowel wall thickening
episode of asparaginase-associated pancreatitis.70 (>3 mm), pneumatosis intestinalis, surrounding soft-tissue
© 2018 Japan Pediatric Society
Pediatric oncologic emergencies 135
Fig. 18 Neutropenic colitis in a 14-year-old boy receiving
b chemotherapy for acute lymphoblastic leukemia. Axial contrast-
enhanced computed tomography showing diffusely thickened
large bowel wall, most prominent in the cecum and ascending
colon (arrow), and less prominently involving the transverse colon
(arrowheads). There is also diffuse mesenteric edema.
Genitourinary emergencies
Acute genitourinary complications in children with malignan-
cies may be caused by pre-renal causes (septic shock), renal
injury (tumor lysis syndrome, chemotherapeutic agents such as
methotrexate or cisplatin, or anti-vascular endothelial growth
factor therapy), or post-renal obstruction.1,81 Children with
acute urinary complications usually present with reduced or
absent urine output with high serum creatinine concentration.
Post-renal obstruction may be caused by large abdominal or
Fig. 17 Acute pancreatitis caused by asparaginase in a 4-year-
old boy with acute lymphoblastic leukemia. (a) Abdominal ultra- pelvic masses (such as Wilms tumor, retroperitoneal sarcoma,
sound showing an enlarged and hypoechoic pancreas (arrow). (b) lymphoma, germ cell tumor, ovarian tumor, adrenal neuroblas-
Axial contrast-enhanced computed tomography showing an toma, and bladder rhabdomyosarcoma) compressing the ureter
enlarged and edematous pancreas (arrow) with a large amount of or bladder. Blood clots from hemorrhagic cystitis can also
ascites (asterisks), consistent with acute pancreatitis.
obstruct the urinary outflow from the bladder.81 Cyclophos-
phamide is a cause of hemorrhagic cystitis. Recent data sug-
edema in the affected region, and free fluid (Fig. 18).74,78 US is gest that BK virus is associated with hemorrhagic cystitis in
as sensitive as CT, avoids radiation exposure, and may provide children undergoing HCT.82 Terminal renal failure is a rare
a more accurate measurement of bowel wall thickness with its event: pediatric malignancies account for only 0.9% of
higher spatial resolution.74,79 Perforation may complicate neu- patients on renal replacement therapy, and many of these
tropenic colitis; this is better evaluated on radiograph or CT. patients have been treated for bilateral Wilms tumor.81
Given that neutropenic colitis can involve the appendix, it Renal and bladder US is the first diagnostic test in patients
may mimic acute appendicitis.80 The incidence of these two with azotemia because it is the least invasive and avoids radia-
diseases is similar and they must be differentiated because tion or use of contrast media. A > 10 mm anteroposterior
most patients with neutropenic colitis recover with medical diameter of the renal pelvis is associated with obstructive
treatment alone, whereas acute appendicitis often requires sur- uropathy (100% sensitivity, 90.3% specificity).83 Contrast-
gery. Distinguishing clinical features in children with neu- enhanced CT may be performed provided renal function is
tropenic colitis include the presence of fever and diarrhea. Of preserved and may show delayed renal parenchymal enhance-
note, appendicitis in children with hematologic malignancies ment (“delayed nephrogram” sign) in patients with obstruc-
may present with atypical clinical findings and an absence of tion, as well as demonstrate the tumor itself or hydro-
characteristic imaging findings. ureteronephrosis (Fig. 19). MR urography can help further
Other differential diagnoses, including infectious enterocoli- evaluation of obstructive uropathy.84 In patients with hemor-
tis (such as pseudomembranous colitis and viral infection), rhagic cystitis, US shows either focal or diffuse bladder wall
and intestinal GVHD are described in detail in the earlier thickening (>3 mm in a distended bladder), often with echo-
section. genic debris (Fig. 20).85
© 2018 Japan Pediatric Society
136 A Handa et al.
a b c d
Fig. 19 Obstructive uropathy in a 12-year-old boy caused by desmoid tumor associated with Gardner syndrome. (a) Coronal
contrast-enhanced computed tomography showing horseshoe kidney, right hydroureteronephrosis (arrows), and a large amount of ascites
(asterisks). (b,c) Coronal balanced fast field echo magnetic resonance imaging showing an intrapelvic tumor (long arrow) with
hydroureteronephrosis (short arrows) invading the distal right ureter and sigmoid colon causing a urinoma (star) and proximal obstruction
(arrowhead). (d) Ureterogram showing a large urinoma (star) in the distal ureter and proximal obstruction (arrowhead). The ascites was
likely urinary ascites.
a b
Fig. 20 Hemorrhagic cystitis caused by cyclophosphamide in a 2-year-old girl with germ cell tumor. (a) Ultrasound showing diffuse bladder
wall thickening (arrows) with echogenic debris (asterisk). (b) Axial T2-weighted and (c) contrast-enhanced fat-saturated coronal T1-weighted
magnetic resonance imaging showing a thick-walled, hyperemic bladder (arrows) with minimal perivesical inflammatory changes.
In conclusion, we have discussed the clinical and imag- approach involving specialists such as pediatricians, pediatric
ing findings in pediatric patients with oncologic emergen- surgeons, pediatric anesthesiologists, pediatric radiation
cies, including a review of recently published studies. We oncologists, and pediatric radiologists is vital in determining
have provided key radiological imaging to illustrate radio- the correct diagnosis and ensuring prompt and appropriate
logical approaches to making diagnoses. A multidisciplinary treatment strategies.
© 2018 Japan Pediatric Society
Pediatric oncologic emergencies 137
16 Brodoefel H, Faul C, Salih H, Vogel W, Fenchel M, Horger
Acknowledgments M. Therapy-related noninfectious complications in patients
The authors would like to thank Dr Gen Nishimura for help with hematologic malignancies: High-resolution computed
tomography findings. J. Thorac. Imaging 2013; 28: W5–11.
with radiological diagnosis. 17 Johkoh T, Muller NL, Taniguchi H et al. Acute interstitial
pneumonia: Thin-section CT findings in 36 patients.
Radiology 1999; 211: 859–63.
Disclosure 18 Kato M, Hirata S, Kikuchi A, Ogawa K, Kishimoto H,
Hanada R. Neuroblastoma presenting with dilated
The authors declare no conflict of interest. cardiomyopathy. Pediatr. Blood Cancer 2008; 50: 391–2.
19 Ingram L, Rivera GK, Shapiro DN. Superior vena cava
syndrome associated with childhood malignancy: Analysis of
Author contributions 24 cases. Med. Pediatr. Oncol. 1990; 18: 476–81.
A.H. wrote this review article. T.N. contributed to the concep- 20 Parish JM, Marschke RF Jr, Dines DE, Lee RE. Etiologic
considerations in superior vena cava syndrome. Mayo Clin.
tion and design of this article, verified the scientific content, Proc. 1981; 56: 407–13.
and edited the manuscript. A.Mak., T.O., Y.M., K.F., T.Ko., 21 Garey CL, Laituri CA, Valusek PA, St Peter SD, Snyder CL.
M.M., Y.K., D.H., T.Ku., C.O., Y.Y., A.Man. reviewed and Management of anterior mediastinal masses in children. Eur.
edited the manuscript. All authors read and approved the final J. Pediatr. Surg. 2011; 21: 310–3.
manuscript. 22 Batra P, Brown K, Collins JD, Holmes EC, Steckel RJ,
Shapiro BJ. Mediastinal masses: Magnetic resonance imaging
in comparison with computed tomography. J. Natl Med.
References Assoc. 1991; 83: 969–74.
23 Perger L, Lee EY, Shamberger RC. Management of children
1 Pizzo PA, Poplack DG, Adamson PC, Blaney SM, Helman L. and adolescents with a critical airway due to compression by
Principles and Practice of Pediatric Oncology, 7th edn. an anterior mediastinal mass. J. Pediatr. Surg. 2008; 43:
Wolters Kluwer, Philadelphia, 2015. 1990–7.
2 Kuhn B, Peters J, Marx GR, Breitbart RE. Etiology, 24 Narang S, Harte BH, Body SC. Anesthesia for patients with a
management, and outcome of pediatric pericardial effusions. mediastinal mass. Anesthesiol. Clin. North America 2001; 19:
Pediatr. Cardiol. 2008; 29: 90–4. 559–79.
3 Arya LS, Narain S, Thavaraj V, Saxena A, Bhargava M. 25 Lee EY, Kritsaneepaiboon S, Arellano CM, Grace RF,
Leukemic pericardial effusion causing cardiac tamponade. Zurakowski D, Boiselle PM. Unsuspected pulmonary emboli
Med. Pediatr. Oncol. 2002; 38: 282–4. in pediatric oncology patients: Detection with MDCT. AJR
4 Tutar HE, Yilmaz E, Atalay S et al. The changing Am. J. Roentgenol. 2010; 194: 1216–22.
aetiological spectrum of pericarditis in children. Ann. Trop. 26 Stein PD, Beemath A, Meyers FA, Skaf E, Sanchez J, Olson
Paediatr. 2002; 22: 251–6. RE. Incidence of venous thromboembolism in patients
5 Roodpeyma S, Sadeghian N. Acute pericarditis in childhood: hospitalized with cancer. Am. J. Med. 2006; 119: 60–8.
A 10-year experience. Pediatr. Cardiol. 2000; 21: 363–7. 27 Monagle P, Adams M, Mahoney M et al. Outcome of
6 Cartagena AM, Levin TL, Issenberg H, Goldman HS. pediatric thromboembolic disease: A report from the Canadian
Pericardial effusion and cardiac hemangioma in the neonate. Childhood Thrombophilia Registry. Pediatr. Res. 2000; 47:
Pediatr. Radiol. 1993; 23: 384–5. 763–6.
7 Goldstein L, Mirvis SE, Kostrubiak IS, Turney SZ. CT 28 Uderzo C, Faccini P, Rovelli A et al. Pulmonary
diagnosis of acute pericardial tamponade after blunt chest thromboembolism in childhood leukemia: 8-years’ experience
trauma. AJR Am. J. Roentgenol. 1989; 152: 739–41. in a pediatric hematology center. J. Clin. Oncol. 1995; 13:
8 Parker MS, Chasen MH, Paul N. Radiologic signs in thoracic 2805–12.
imaging: Case-based review and self-assessment module. AJR 29 Lee EY, Zurakowski D, Diperna S, d’Almeida Bastos M,
Am. J. Roentgenol. 2009; 192 (3 Suppl): S34–48. Strauss KJ, Boiselle PM. Parenchymal and pleural
9 Restrepo CS, Lemos DF, Lemos JA et al. Imaging findings in abnormalities in children with and without pulmonary
cardiac tamponade with emphasis on CT. Radiographics embolism at MDCT pulmonary angiography. Pediatr. Radiol.
2007; 27: 1595–610. 2010; 40: 173–81.
10 Breen JF. Imaging of the pericardium. J. Thorac. Imaging 30 Stein PD, Chenevert TL, Fowler SE et al. Gadolinium-
2001; 16: 47–54. enhanced magnetic resonance angiography for pulmonary
11 Coley BD. Caffey’s Pediatric Diagnostic Imaging. Saunders, embolism: A multicenter prospective study (PIOPED III).
Philadelphia, PA, 2013. Ann. Intern. Med. 2010; 152 : w142–3.
12 Caillot D, Mannone L, Cuisenier B, Couaillier JF. Role of 31 Long SS, Prober CG, Fischer M. Principles and Practice of
early diagnosis and aggressive surgery in the management of Pediatric Infectious Diseases, 5th edn. Elsevier, Philadelphia,
invasive pulmonary aspergillosis in neutropenic patients. Clin. 2018.
Microbiol. Infect. 2001; 7 (Suppl 2): 54–61. 32 Elicker BM, Webb WR. Fundamentals of High-Resolution
13 Heggen J, West C, Olson E et al. Diffuse alveolar hemorrhage Lung CT. Wolters Kluwer, Philadelphia, PA, 2013.
in pediatric hematopoietic cell transplant patients. Pediatrics 33 Eslamy HK, Newman B. Pneumonia in normal and
2002; 109: 965–71. immunocompromised children: An overview and update.
14 Ben-Abraham R, Paret G, Cohen R et al. Diffuse alveolar Radiol. Clin. North Am. 2011; 49: 895–920.
hemorrhage following allogeneic bone marrow transplantation 34 Osborne D. Radiologic appearance of viral disease of the
in children. Chest 2003; 124: 660–4. lower respiratory tract in infants and children. AJR Am. J.
15 Furuya ME, Rodriguez-Velasco A, Rodriguez-Zepeda MC Roentgenol. 1978; 130: 29–33.
et al. Unsuspected lung pathology in autopsies of children 35 Han SB, Kim SK, Bae EY et al. Clinical features and
with cancer. Rev. Invest. Clin. 2017; 69: 28–32. prognosis of invasive pulmonary aspergillosis in Korean
© 2018 Japan Pediatric Society
138 A Handa et al.
children with hematologic/oncologic diseases. J. Korean Med. neuroblastoma in an infant: A rare case and review of the
Sci. 2015; 30: 1121–8. literature. J. Pediatr. Hematol. Oncol. 2012; 34: 635–7.
36 Thomas KE, Owens CM, Veys PA, Novelli V, Costoli V. The 55 Fawkner-Corbett DW, Howell L, Pizer BL, Dominici C,
radiological spectrum of invasive aspergillosis in children: A McDowell HP, Losty PD. Wilms’ tumor: Lessons and
10-year review. Pediatr. Radiol. 2003; 33: 453–60. outcomes – a 25-year single center UK experience. Pediatr.
37 Tanaka N, Kunihiro Y, Yujiri T et al. High-resolution Hematol. Oncol. 2014; 31: 400–8.
computed tomography of chest complications in patients 56 Meyers RL, Maibach R, Hiyama E et al. Risk-stratified
treated with hematopoietic stem cell transplantation. Jpn. J. staging in paediatric hepatoblastoma: A unified analysis from
Radiol. 2011; 29: 229–35. the Children’s Hepatic tumors International Collaboration.
38 Schmid I, Stachel D, Pagel P, Albert MH. Incidence, Lancet Oncol. 2017; 18: 122–31.
predisposing factors, and outcome of engraftment syndrome in 57 Iwata A, Matsubara K, Umemoto Y, Hashimoto K, Fukaya T.
pediatric allogeneic stem cell transplant recipients. Biol. Blood Spontaneous rupture of benign ovarian cystic teratoma in a
Marrow Transplant. 2008; 14: 438–44. premenarcheal girl. J. Pediatr. Adolesc. Gynecol. 2009; 22:
39 Pena E, Souza CA, Escuissato DL et al. Noninfectious e121–3.
pulmonary complications after hematopoietic stem cell 58 Duigenan S, Oliva E, Lee SI. Ovarian torsion: Diagnostic
transplantation: Practical approach to imaging diagnosis. features on CT and MRI with pathologic correlation. AJR Am.
Radiographics 2014; 34: 663–83. J. Roentgenol. 2012; 198: W122–31.
40 Keates-Baleeiro J, Moore P, Koyama T, Manes B, Calder C, 59 Jacobsohn DA. Acute graft-versus-host disease in children.
Frangoul H. Incidence and outcome of idiopathic pneumonia Bone Marrow Transplant. 2008; 41: 215–21.
syndrome in pediatric stem cell transplant recipients. Bone 60 Billingham RE. The biology of graft-versus-host reactions.
Marrow Transplant. 2006; 38: 285–9. Harvey Lect. 1966; 62: 21–78.
41 Chan PW, Muridan R, Debruyne JA. Bronchiolitis obliterans 61 Ferrara JL, Deeg HJ. Graft-versus-host disease. N. Engl. J.
in children: Clinical profile and diagnosis. Respirology 2000; Med. 1991; 324: 667–74.
5: 369–75. 62 Lee JH, Lim GY, Im SA, Chung NG, Hahn ST.
42 Lynch DA, Hay T, Newell JD Jr, Divgi VD, Fan LL. Gastrointestinal complications following hematopoietic stem
Pediatric diffuse lung disease: Diagnosis and classification cell transplantation in children. Korean J. Radiol. 2008; 9:
using high-resolution CT. AJR Am. J. Roentgenol. 1999; 173: 449–57.
713–8. 63 Donnelly LF, Morris CL. Acute graft-versus-host disease in
43 Mathew P, Bozeman P, Krance RA, Brenner MK, Heslop HE. children: Abdominal CT findings. Radiology 1996; 199: 265–
Bronchiolitis obliterans organizing pneumonia (BOOP) in 8.
children after allogeneic bone marrow transplantation. Bone 64 Lubner MG, Menias CO, Agrons M et al. Imaging of
Marrow Transplant. 1994; 13: 221–3. abdominal and pelvic manifestations of graft-versus-host
44 Polverosi R, Maffesanti M, Dalpiaz G. Organizing pneumonia: disease after hematopoietic stem cell transplant. AJR Am. J.
Typical and atypical HRCT patterns. Radiol. Med. 2006; 111: Roentgenol. 2017; 209: 33–45.
202–12. 65 Day DL, Ramsay NK, Letourneau JG. Pneumatosis intestinalis
45 Zinter MS, Melton A, Sabnis AJ et al. Pulmonary veno- after bone marrow transplantation. AJR Am. J. Roentgenol.
occlusive disease in a pediatric hematopoietic stem cell 1988; 151: 85–7.
transplant patient: A cautionary tale. Leuk. Lymphoma 2018; 66 Han W, Wang HM. Refractory diarrhea: A paraneoplastic
59: 1494–7. syndrome of neuroblastoma. World J. Gastroenterol. 2015; 21:
46 Resten A, Maitre S, Humbert M et al. Pulmonary 7929–32.
hypertension: CT of the chest in pulmonary venoocclusive 67 Korhonen K, Lovvorn HN 3rd, Koyama T et al. Incidence,
disease. AJR Am. J. Roentgenol. 2004; 183: 65–70. risk factors, and outcome of pneumatosis intestinalis in
47 LaQuaglia MP, Stolar CJ, Krailo M et al. The role of surgery pediatric stem cell transplant recipients. Pediatr. Blood
in abdominal non-Hodgkin’s lymphoma: Experience from the Cancer 2012; 58: 616–20.
Children’s Cancer Study Group. J. Pediatr. Surg. 1992; 27: 68 Olson DE, Kim YW, Ying J, Donnelly LF. CT predictors for
230–5. differentiating benign and clinically worrisome pneumatosis
48 Gupta H, Davidoff AM, Pui CH, Shochat SJ, Sandlund JT. intestinalis in children beyond the neonatal period. Radiology
Clinical implications and surgical management of 2009; 253: 513–9.
intussusception in pediatric patients with Burkitt lymphoma. J. 69 Kearney SL, Dahlberg SE, Levy DE, Voss SD, Sallan SE,
Pediatr. Surg. 2007; 42: 998–1001. Silverman LB. Clinical course and outcome in children with
49 Biko DM, Anupindi SA, Hernandez A, Kersun L, Bellah R. acute lymphoblastic leukemia and asparaginase-associated
Childhood Burkitt lymphoma: Abdominal and pelvic imaging pancreatitis. Pediatr. Blood Cancer 2009; 53: 162–7.
findings. AJR Am. J. Roentgenol. 2009; 192: 1304–15. 70 Wolthers BO, Frandsen TL, Baruchel A et al. Asparaginase-
50 Strouse PJ, DiPietro MA, Saez F. Transient small-bowel associated pancreatitis in childhood acute lymphoblastic
intussusception in children on CT. Pediatr. Radiol. 2003; 33: leukaemia: An observational Ponte di Legno Toxicity
316–20. Working Group study. Lancet Oncol. 2017; 18: 1238–48.
51 Kim JH. US features of transient small bowel intussusception 71 Liu C, Yang W, Devidas M et al. Clinical and genetic risk
in pediatric patients. Korean J. Radiol. 2004; 5: 178–84. factors for acute pancreatitis in patients with acute
52 Munden MM, Bruzzi JF, Coley BD, Munden RF. Sonography lymphoblastic leukemia. J. Clin. Oncol. 2016; 34: 2133–40.
of pediatric small-bowel intussusception: Differentiating 72 Abaji R, Gagne V, Xu CJ et al. Whole-exome sequencing
surgical from nonsurgical cases. AJR Am. J. Roentgenol. 2007; identified genetic risk factors for asparaginase-related
188: 275–9. complications in childhood ALL patients. Oncotarget 2017; 8:
53 Goldberg SR, Godder K, Lanning DA. Successful treatment of 43752–67.
a bowel perforation after chemotherapy for Burkitt lymphoma. 73 Raja RA, Schmiegelow K, Albertsen BK et al. Asparaginase-
J. Pediatr. Surg. 2007; 42 : E1–3. associated pancreatitis in children with acute lymphoblastic
54 Honda S, Miyagi H, Minato M, Kubota KC, Okada T. leukaemia in the NOPHO ALL2008 protocol. Br. J.
Hemorrhagic shock due to spontaneous rupture of adrenal Haematol. 2014; 165: 126–33.
© 2018 Japan Pediatric Society
Pediatric oncologic emergencies 139
74 McCarville MB, Adelman CS, Li C et al. Typhlitis in with typhilitis. J. Pediatr. Surg. 2005; 40: 214–9; discussion
childhood cancer. Cancer 2005; 104: 380–7. 9–20.
75 Fike FB, Mortellaro V, Juang D, St Peter SD, Andrews WS, 81 Rossi R, Kleta R, Ehrich JH. Renal involvement in children
Snyder CL. Neutropenic colitis in children. J. Surg. Res. 2011; with malignancies. Pediatr. Nephrol. 1999; 13: 153–62.
170: 73–6. 82 Pinto M, Dobson S. BK and JC virus: A review. J. Infect.
76 Davila ML. Neutropenic Enterocolitis. Curr. Treat. Options 2014; 68 (Suppl 1): S2–8.
Gastroenterol. 2006; 9: 249–55. 83 Tsai TC, Lee HC, Huang FY. The size of the renal pelvis on
77 Rodrigues FG, Dasilva G, Wexner SD. Neutropenic ultrasonography in children. J. Clin. Ultrasound 1989; 17:
enterocolitis. World J. Gastroenterol. 2017; 23: 42–7. 647–51.
78 Kirkpatrick ID, Greenberg HM. Gastrointestinal complications 84 Dickerson EC, Dillman JR, Smith EA, DiPietro MA,
in the neutropenic patient: Characterization and differentiation Lebowitz RL, Darge K. Pediatric MR urography: Indications,
with abdominal CT. Radiology 2003; 226: 668–74. techniques, and approach to review. Radiographics 2015; 35:
79 McCarville MB. Evaluation of typhlitis in children: CT versus 1208–30.
US. Pediatr. Radiol. 2006; 36: 890–1. 85 McCarville MB, Hoffer FA, Gingrich JR, Jenkins JJ 3rd.
80 Hobson MJ, Carney DE, Molik KA et al. Appendicitis in Imaging findings of hemorrhagic cystitis in pediatric oncology
childhood hematologic malignancies: Analysis and comparison patients. Pediatr. Radiol. 2000; 30: 131–8.
© 2018 Japan Pediatric Society
You might also like
- Clinical Cases in Chronic Thromboembolic Pulmonary HypertensionFrom EverandClinical Cases in Chronic Thromboembolic Pulmonary HypertensionWilliam R. AugerNo ratings yet
- Pitfalls of EmphysemaDocument7 pagesPitfalls of EmphysemaWahyu Puspita IrjayantiNo ratings yet
- PDF To WordDocument17 pagesPDF To Wordanisa ghinaNo ratings yet
- Congestive Heart FailureDocument9 pagesCongestive Heart FailureMaria Aprilia DiniNo ratings yet
- Emergency Department Guide to Diagnosing and Treating Pleural DiseaseDocument25 pagesEmergency Department Guide to Diagnosing and Treating Pleural DiseaseEdgardo Vargas AlvarezNo ratings yet
- Restrepo 2012Document15 pagesRestrepo 2012catalinaNo ratings yet
- Airways in Mediastinal Mass PDFDocument9 pagesAirways in Mediastinal Mass PDFHarish BhatNo ratings yet
- 52 Vascular FistulaeDocument6 pages52 Vascular FistulaeVictor PazNo ratings yet
- Blunt Thoracic TraumaDocument3 pagesBlunt Thoracic TraumaNetta LionoraNo ratings yet
- Edema Pulmo JurnalDocument14 pagesEdema Pulmo JurnalOlivia Chandra DeviNo ratings yet
- 2010 Hemorragia Alveolar DifusaDocument8 pages2010 Hemorragia Alveolar DifusaCecilia Requejo TelloNo ratings yet
- Develpmental Lung AnomaliDocument11 pagesDevelpmental Lung AnomaliSiti Amalia PratiwiNo ratings yet
- Clinical Imaging: Michela Gabelloni, Lorenzo Faggioni, Sandra Accogli, Giacomo Aringhieri, Emanuele NeriDocument12 pagesClinical Imaging: Michela Gabelloni, Lorenzo Faggioni, Sandra Accogli, Giacomo Aringhieri, Emanuele NeriJavier Araya CancinoNo ratings yet
- Review Article Thoracic Involvement in Systemic Primary Vasculitis: Radio Logical Patterns and Follow UpDocument12 pagesReview Article Thoracic Involvement in Systemic Primary Vasculitis: Radio Logical Patterns and Follow UpLiza EgudinsNo ratings yet
- OMICS Journal of Radiology: Pulmonary Hypertension Secondary To Cardiac Hydatid Cyst EmbolismDocument2 pagesOMICS Journal of Radiology: Pulmonary Hypertension Secondary To Cardiac Hydatid Cyst EmbolismAgusNo ratings yet
- ABIGAIL 2010_Diffuse Alveolar HemorrhageDocument10 pagesABIGAIL 2010_Diffuse Alveolar HemorrhageRafael JustinoNo ratings yet
- TRAUMA Pulmones, Traquea y EsofagoDocument14 pagesTRAUMA Pulmones, Traquea y EsofagosantigarayNo ratings yet
- Idiopathic Pulmonary FibrosisDocument13 pagesIdiopathic Pulmonary FibrosisRaul David Maldonado LearyNo ratings yet
- Pericardio Dis Pericarditis - Recurr - Constric RNMC CMR JACC 2023Document16 pagesPericardio Dis Pericarditis - Recurr - Constric RNMC CMR JACC 2023Percy DurandNo ratings yet
- Fpi Nejm Clincal Practice 2018Document13 pagesFpi Nejm Clincal Practice 2018cjonderedsonNo ratings yet
- Caso Clinico Sp3Document4 pagesCaso Clinico Sp3eduardoNo ratings yet
- Radiological Findings of Unilateral Tuberculous LuDocument7 pagesRadiological Findings of Unilateral Tuberculous LuSarah watiNo ratings yet
- Jurnal 2Document0 pagesJurnal 2Seftiana SaftariNo ratings yet
- Jurnal Radio Inggris TalDocument17 pagesJurnal Radio Inggris TalimamattamamiNo ratings yet
- Cyst and Cystlike Lung LesionsDocument57 pagesCyst and Cystlike Lung LesionsAna StankovicNo ratings yet
- Interpretando RX PediatricoDocument10 pagesInterpretando RX PediatricosuzukishareNo ratings yet
- CH 74Document3 pagesCH 74alimonyNo ratings yet
- Pulmonary Aspergillosis Imaging Findings and DiagnosisDocument9 pagesPulmonary Aspergillosis Imaging Findings and DiagnosisPutri PrameswariNo ratings yet
- Journal Radiologi 3Document14 pagesJournal Radiologi 3WinayNayNo ratings yet
- Cardiac TamponadeDocument18 pagesCardiac Tamponadeyelsinosmin romeroalvaradoNo ratings yet
- Imaging findings of pulmonary edema: Part 1. Cardiogenic pulmonary edema and acute respiratory distress syndromeDocument11 pagesImaging findings of pulmonary edema: Part 1. Cardiogenic pulmonary edema and acute respiratory distress syndromeOlivia Chandra DeviNo ratings yet
- SURGICAL PROCEDURES AND COMPLICATIONSDocument55 pagesSURGICAL PROCEDURES AND COMPLICATIONSNikxyNo ratings yet
- Stenosis Pulmonal.Document21 pagesStenosis Pulmonal.syelNo ratings yet
- Tetralogy of Fallot and Its VariantsDocument7 pagesTetralogy of Fallot and Its VariantssofiaNo ratings yet
- Jurnal Kedokteran Dan Kesehatan IndonesiaDocument10 pagesJurnal Kedokteran Dan Kesehatan IndonesiaIrna WatiNo ratings yet
- Ehs 372Document13 pagesEhs 372Rocio BeteluNo ratings yet
- Pulmonary Edema - A Pictorial Review of Imaging Manifestations and Current Understanding of Mechanisms of DiseaseDocument14 pagesPulmonary Edema - A Pictorial Review of Imaging Manifestations and Current Understanding of Mechanisms of Diseaseoralposter PIPKRA2023No ratings yet
- 521 FullDocument11 pages521 FulljhordyafsNo ratings yet
- Obstructive Shock Pediatric - Journal PDFDocument3 pagesObstructive Shock Pediatric - Journal PDFGerald SweinslionNo ratings yet
- Surgical Therapy For Necrotizing Pneumonia and Lung Gangrene PDFDocument6 pagesSurgical Therapy For Necrotizing Pneumonia and Lung Gangrene PDFmichael24kNo ratings yet
- Saddle Embolism in The Pulmonary Artery BifurcationDocument3 pagesSaddle Embolism in The Pulmonary Artery BifurcationAtiquzzaman RinkuNo ratings yet
- Standard Treatment Guidelines Interventional Radiology: Ministry of Health & Family Welfare Govt. of IndiaDocument75 pagesStandard Treatment Guidelines Interventional Radiology: Ministry of Health & Family Welfare Govt. of IndiaKaustubhNo ratings yet
- Thoracentesis - ClinicalKey (1) (01-11)Document11 pagesThoracentesis - ClinicalKey (1) (01-11)jose luisNo ratings yet
- ANGIOSARCOMA A Rare Cause of Pleural MalignancyDocument3 pagesANGIOSARCOMA A Rare Cause of Pleural MalignancyInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Bacterial Infections of the CNSDocument13 pagesBacterial Infections of the CNSFernanda ChNo ratings yet
- Minimally invasive treatment of hemothoraxDocument8 pagesMinimally invasive treatment of hemothoraxBenny KurniawanNo ratings yet
- CDH Modern MX PDFDocument12 pagesCDH Modern MX PDFThia SanjayaNo ratings yet
- Pulmonary Fibrosis in Sytemic Sclerosis Diagnosis and ManagementDocument5 pagesPulmonary Fibrosis in Sytemic Sclerosis Diagnosis and ManagementAnthoNo ratings yet
- Cureus 0012 00000011411Document5 pagesCureus 0012 00000011411Marsya Yulinesia LoppiesNo ratings yet
- Management of Primary Spontaneous PneumothoraxDocument6 pagesManagement of Primary Spontaneous PneumothoraxPendragon ArthurNo ratings yet
- 080508-148 Syphilitc Aneurys Case Report PDFDocument4 pages080508-148 Syphilitc Aneurys Case Report PDFCarol SantosNo ratings yet
- Hypoxia and Complications of Oxygenation in Extracorporeal Membrane OxygenationDocument7 pagesHypoxia and Complications of Oxygenation in Extracorporeal Membrane Oxygenationjq4rhbh2pzNo ratings yet
- Update On The Diagnosis and Treatment of Tracheal and Bronchial InjuryDocument7 pagesUpdate On The Diagnosis and Treatment of Tracheal and Bronchial InjurymeiutaNo ratings yet
- Congenital Lobar EmphysemaDocument3 pagesCongenital Lobar Emphysemamadimadi11No ratings yet
- Approach to a Patient with Haemoptysis and Normal Chest X-RayDocument9 pagesApproach to a Patient with Haemoptysis and Normal Chest X-Raydoc_next_doorNo ratings yet
- ACUTE PULMONARY EMBOLISMDocument15 pagesACUTE PULMONARY EMBOLISMPuguh FebriantoNo ratings yet
- Anesthetic Management of Patients With An Anterior Mediastinal Mass: Continuing Professional DevelopmentDocument15 pagesAnesthetic Management of Patients With An Anterior Mediastinal Mass: Continuing Professional DevelopmentRicardo VargasNo ratings yet
- Imaging in Non-Cardiac Chest EmergenciesDocument8 pagesImaging in Non-Cardiac Chest EmergenciesAta07No ratings yet
- Lapierre 2016Document11 pagesLapierre 2016riswandarizkiNo ratings yet
- Young Man With Cardiac Arrest Secondary To Undiagnosed Mediastinal MassDocument4 pagesYoung Man With Cardiac Arrest Secondary To Undiagnosed Mediastinal MassPxPPxH ChanNo ratings yet
- Triple Artrodesis TobilloDocument17 pagesTriple Artrodesis TobilloIsrael CucsNo ratings yet
- Perio-Prosthodontic Pontic Site Management, Part I: Pontic Designs and Their Current ApplicationsDocument12 pagesPerio-Prosthodontic Pontic Site Management, Part I: Pontic Designs and Their Current ApplicationsFelipe Castro100% (4)
- Pro Pro: Guard Guard LyoDocument4 pagesPro Pro: Guard Guard LyoLaiNo ratings yet
- Nature of Request No. of Cases Amount: Summary of Daily Cases Received Nueva Vizcaya Branch AUGUST 8, 2019Document25 pagesNature of Request No. of Cases Amount: Summary of Daily Cases Received Nueva Vizcaya Branch AUGUST 8, 2019Aileen EddubaNo ratings yet
- Vascular ImagingDocument277 pagesVascular ImagingJean-Philippe BinderNo ratings yet
- Max. MAJOR CONNECTORSDocument51 pagesMax. MAJOR CONNECTORSأمال داودNo ratings yet
- Hysteroscopes From KARL STORZ: Diagnostic and Operative Solutions For "Office Hysteroscopy"Document16 pagesHysteroscopes From KARL STORZ: Diagnostic and Operative Solutions For "Office Hysteroscopy"Marcus MenezesNo ratings yet
- UntitledDocument258 pagesUntitledIryna ZapekaNo ratings yet
- Leibinger Universal Plating System Reference GuideDocument92 pagesLeibinger Universal Plating System Reference GuidebeetgecsgoNo ratings yet
- Epidural Intraspinal Anticoagulation Guidelines - UKDocument9 pagesEpidural Intraspinal Anticoagulation Guidelines - UKjoshNo ratings yet
- Coronary CT Angiography ExamDocument4 pagesCoronary CT Angiography ExamCarlos F Muñoz NúñezNo ratings yet
- Fisher & Paykel MR 850Document20 pagesFisher & Paykel MR 850NithinBesentNNo ratings yet
- Baroreceptor Modulation of The Cardiovascular System, Pain, Consciousness, and CognitionDocument51 pagesBaroreceptor Modulation of The Cardiovascular System, Pain, Consciousness, and CognitionPatoNo ratings yet
- ABSTRACT TEVAR - Achmad Ismail S PutraDocument2 pagesABSTRACT TEVAR - Achmad Ismail S PutraPutra AchmadNo ratings yet
- Checklist For Changing Central Line DressingDocument1 pageChecklist For Changing Central Line DressingShuchi RaiNo ratings yet
- Year 9 Basic First Aid Exam ResusDocument2 pagesYear 9 Basic First Aid Exam ResusJames VincentNo ratings yet
- Case-Based Review in Critical Care MedicineDocument424 pagesCase-Based Review in Critical Care Medicinecristian gutierrezNo ratings yet
- A5 Brochure - Revise - Centre Pinning - With IndoscopeDocument32 pagesA5 Brochure - Revise - Centre Pinning - With IndoscopeGerman DrumsNo ratings yet
- Definition, Classification, Etiology, and Pathophysiology of Shock in Adults - UpToDateDocument15 pagesDefinition, Classification, Etiology, and Pathophysiology of Shock in Adults - UpToDateHartanto SantosoNo ratings yet
- Weber 2020Document11 pagesWeber 2020Yefry Onil Santana MarteNo ratings yet
- Legg CALVE PERVE DISEASEDocument7 pagesLegg CALVE PERVE DISEASEYussika FernandaNo ratings yet
- Contoh Pico Terbaru-Blok EndokrinDocument3 pagesContoh Pico Terbaru-Blok EndokrinIndra ArdiansyahNo ratings yet
- Obs-UM - Paper - 1Document16 pagesObs-UM - Paper - 1Muhammad Abbas AliNo ratings yet
- SBAR Doris BowmanDocument2 pagesSBAR Doris BowmanM.I Dia Izibelle F. AMENAMENNo ratings yet
- HEMMORRHOIDECTOMYDocument16 pagesHEMMORRHOIDECTOMYLily CentenoNo ratings yet
- The Art of SurgeryDocument3 pagesThe Art of SurgerymuzualyNo ratings yet
- Examining the Abdomen Effectively in Under 10 MinutesDocument21 pagesExamining the Abdomen Effectively in Under 10 MinutesnouraNo ratings yet
- Complications in Corneal Laser SurgeryDocument233 pagesComplications in Corneal Laser SurgeryGreg LopezNo ratings yet
- The Right Choice: Smith&nephewDocument4 pagesThe Right Choice: Smith&nephewapi-19808945No ratings yet