Professional Documents
Culture Documents
Alkanes
Alkanes
Uploaded by
b52352986Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Alkanes
Alkanes
Uploaded by
b52352986Copyright:
Available Formats
Alkanes
Alkanes
>> simplest family of organic chemistry
>> they are hydro carbons : they contain hydrogen and oxygen only
>> they have a carbon - carbon bond ( single bonds )
>> general formula is CnH2n+2
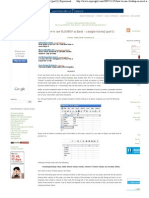
Molecular Structural
Displayed formula formula
Alkane formula
Methane CH4
CH4
Ethane C2H6
CH3CH3
Propane C3H8
CH3CH2CH3
Butane C4 H10 CH3CH2CH2CH3
Pentane C5H12 CH3CH2CH2CH2CH3
Structural isomers
Structural isomers : molecules with the same molecular formula but
different displayed and structural formula
P h y s i c a l p ro p e r t ie s
>> F i rst 4 a l k a n e s a re g a s e s at ro o m te mp, t h e re st a re l i q u id , s o l id
st a r t f ro m c a r b o n 18
» As s i ze i n c re a s e b o i l i n g p o i nt a l s o i n c re a s e
» As s i ze i n c re a s e i nte r m o l e c u l a r fo rc e s o f at t r a ct i o n a l s o i n c re a s e
→ u n re a ct i ve b e c a u s e i t h a s c a r b o n - C a r b o n b o n d
C h e m i c a l p ro p e r t ie s
>>I n e r t
→ t h ey a l l b e h a ve t h e s a m e w ay
C H E M I CA L R E ACT I ON S
C o m b u st i o n
1. C o mp l ete c o m b u st i o n
W h e n e n o u g h ox yg e n i s p re s e nt fo r t h e a l k a n e to b e b u r n e d f u l l y to
g i ve c a r b o n d i ox ide a n d w ate r
C H4 + 2O2 = CO2 + H2O
2. I n c o mp l ete C o m b u st i o n
W h e n t h e a m o u nt o f ox yg e n i s l i m i te d s o t h e i n c o mp l ete c o m b u st i o n
o f h yd ro c a r b o n s h app e n a n d c a r b o n m o n ox ide a n d w ate r a re g i ve n o u t
2C2H6 + 5O2 = 4 CO +6H2O
R e a ct i o n s w i t h h a l o g e n s
>> a l k a n e s re a ct w i t h h a l o g e n s i n t h e p re s e n c e o f u l t r a v i o l et l i g ht
>> a n h yd ro g e n ato m i n t h e a l k a n e i s d i sp l a c e d b y a h a l o g e n ato m
>> t h i s i s k n o w n a s s u b st i t u t i o n re a ct i o n b e c a u s e a n ato m i s b e i n g
s u b st i t u te d b y a d i f fe re nt o n e
C H 4 . + C l 2 = C H 3 C l + H C l
Mono - substitution
When only one hydrogen atom in the alkanes is replaced by
halogen atom
You might also like
- User Requirements For A Water For Injection SystemDocument42 pagesUser Requirements For A Water For Injection SystemJordi Sos Navarro67% (3)
- TroubleshootingDocument296 pagesTroubleshootingmoein100% (1)
- ReactiontypesDocument15 pagesReactiontypesapi-483662721No ratings yet
- 32 - 3 - New Orleans - 08-87 - 0255Document5 pages32 - 3 - New Orleans - 08-87 - 0255Abdul AzizNo ratings yet
- Polymer - : CH CH CH CHDocument2 pagesPolymer - : CH CH CH CHarch360No ratings yet
- Organic Chem IIDocument55 pagesOrganic Chem IIAbdulhamid AbdulwaasiNo ratings yet
- Monografia Captopril ConnorsDocument3 pagesMonografia Captopril ConnorsAngelica MedinaNo ratings yet
- 23 - 3 - Miami Beach - 09-78 - 0160Document8 pages23 - 3 - Miami Beach - 09-78 - 0160lam2289No ratings yet
- Gpo Price Csfti Price (S) $Document5 pagesGpo Price Csfti Price (S) $maxwellstreetguyNo ratings yet
- Introduction To EvDocument21 pagesIntroduction To EvJulio TuestaNo ratings yet
- Carbon Rsbitw Detailed NotesDocument21 pagesCarbon Rsbitw Detailed NotesAnshulNo ratings yet
- Butadiene PproductionDocument5 pagesButadiene PproductionYoteshYadavNo ratings yet
- Petroleum Coke Handling Problems H. Great Carbon Corporation New NDocument9 pagesPetroleum Coke Handling Problems H. Great Carbon Corporation New NEumarielys EspinozaNo ratings yet
- Lab 2: Extraction 2 / 3 / 2 0 1 4 Jeffrey Dixon Partner: Cedrian HodgeDocument4 pagesLab 2: Extraction 2 / 3 / 2 0 1 4 Jeffrey Dixon Partner: Cedrian HodgedraykidNo ratings yet
- Nanofiltration To Separate Salts From H2S Scrubber Solutions Continuous Microfiltration Process SelectedDocument1 pageNanofiltration To Separate Salts From H2S Scrubber Solutions Continuous Microfiltration Process SelectedAditya RahmatNo ratings yet
- Alkynes: Val Joseph de Guzman Marx Lennin CabalticanDocument36 pagesAlkynes: Val Joseph de Guzman Marx Lennin CabalticanMarx Lennin Cabaltican0% (1)
- Ali 1983Document32 pagesAli 1983Andres Rengifo BotinaNo ratings yet
- Thermodynamic Study of Ice and Clathrate Hydrates : Chem.Document10 pagesThermodynamic Study of Ice and Clathrate Hydrates : Chem.Shurooq TaibNo ratings yet
- 13 - 4 - New York - 09-69 - 0121 PDFDocument9 pages13 - 4 - New York - 09-69 - 0121 PDFDedik DermadyNo ratings yet
- Syn-So: 2/ Pleasant Hills Sewagetreatment Plant, 1222 Cochran Mill Road, Pittsburgh, Pa.Document9 pagesSyn-So: 2/ Pleasant Hills Sewagetreatment Plant, 1222 Cochran Mill Road, Pittsburgh, Pa.Nurliyana Syahirah ShabriNo ratings yet
- Isolation and Structure Elucidation Tannins: ApplDocument4 pagesIsolation and Structure Elucidation Tannins: ApplElsa Fernita ManullangNo ratings yet
- The Use Sorbents To Remove Hydrogen Sulfide Coal T. L. LA: From Gases AtimtayDocument8 pagesThe Use Sorbents To Remove Hydrogen Sulfide Coal T. L. LA: From Gases Atimtayngnm0No ratings yet
- Estimating Methane Content of Bituminous Coalbeds From AdsorptionDocument26 pagesEstimating Methane Content of Bituminous Coalbeds From AdsorptionAdhitya KuswantoroNo ratings yet
- Organic Chemistry Some Basic Principles and Techniques 100 Questions With Solution 0 2023 01 10 095917Document31 pagesOrganic Chemistry Some Basic Principles and Techniques 100 Questions With Solution 0 2023 01 10 095917rashmiNo ratings yet
- Effect Oxygen Compounds Addition O N The Hydrocracking of AlkylpbenolsDocument4 pagesEffect Oxygen Compounds Addition O N The Hydrocracking of AlkylpbenolsShakir AbbasNo ratings yet
- Recuperción de Níquel ECOTECDocument11 pagesRecuperción de Níquel ECOTECJesús RiberaNo ratings yet
- 23 - 4 - Miami Beach - 09-78 - 0072Document9 pages23 - 4 - Miami Beach - 09-78 - 0072adrian2009-2020No ratings yet
- 15 - 2 - Washington DC - 09-71 - 0150Document8 pages15 - 2 - Washington DC - 09-71 - 0150Matias MancillaNo ratings yet
- Chretien To Trudeau 71.07Document6 pagesChretien To Trudeau 71.07Russell DiaboNo ratings yet
- Exp 5 and 6Document6 pagesExp 5 and 6Aditya Singh RajputNo ratings yet
- Metode Penentuan NAD, NADH, NADP, NADPHDocument28 pagesMetode Penentuan NAD, NADH, NADP, NADPHYovi AviantoNo ratings yet
- Covellite To DigeniteDocument7 pagesCovellite To DigeniteMiizoreNo ratings yet
- 29 6 Philadelphia 08-84 0066Document13 pages29 6 Philadelphia 08-84 0066Azza M. ElnenaeyNo ratings yet
- Cyanide Geochemistry and Detoxification RegulationsDocument18 pagesCyanide Geochemistry and Detoxification RegulationsEduardo calvo gilNo ratings yet
- Ranjeet ShahiDocument11 pagesRanjeet Shahisabhari_ram50% (2)
- 18 2 Dallas 04-73 0142Document15 pages18 2 Dallas 04-73 0142ea88b5No ratings yet
- Metal Extraction (Recovery Systems) PDFDocument13 pagesMetal Extraction (Recovery Systems) PDFMary JohnsonNo ratings yet
- Chemical Methods USE in Marine Environmental Monitoring: Commission ManualsDocument56 pagesChemical Methods USE in Marine Environmental Monitoring: Commission ManualsabufetehyNo ratings yet
- Determination of Carbohydrates by Anion Exchange Chromatography With Pulsed Amperometric DetectionDocument16 pagesDetermination of Carbohydrates by Anion Exchange Chromatography With Pulsed Amperometric DetectionXYZUSPNo ratings yet
- 16 4 Boston 04-72 0070 PDFDocument9 pages16 4 Boston 04-72 0070 PDFGurusangmeshHiremathNo ratings yet
- Aroma Ti CityDocument33 pagesAroma Ti Citymidhungbabu88No ratings yet
- Ranjeet ShahiDocument11 pagesRanjeet Shahisabhari_ram100% (1)
- Diffusion Modeling of The Carburization Process: That They InfluenceDocument2 pagesDiffusion Modeling of The Carburization Process: That They InfluenceWahyuNo ratings yet
- Thesis On Wastewater TreatmentDocument5 pagesThesis On Wastewater Treatmentaflozmfxxranis100% (2)
- Polymer Synthesis: "I Am Inclined To Think That TheDocument21 pagesPolymer Synthesis: "I Am Inclined To Think That ThearobaidiNo ratings yet
- Lab Activity 1 1Document20 pagesLab Activity 1 1zariffah sandroNo ratings yet
- SAT Subject Chemistry SummaryDocument25 pagesSAT Subject Chemistry SummaryYoonho LeeNo ratings yet
- 3 AlkenesDocument22 pages3 AlkenesAung QinkangNo ratings yet
- Flotation Li Minerals - Lombe - WC - 1983 - PHD - ThesisDocument259 pagesFlotation Li Minerals - Lombe - WC - 1983 - PHD - ThesisMaria José FuturoNo ratings yet
- Unit 2 Lesson 1 AnswersDocument5 pagesUnit 2 Lesson 1 AnswersShazara MohammedNo ratings yet
- CEMENT and CONCRETE RESEARCH. Vol. 4, Pp. 69-76, 1974. Pergamon Press, Inc. Printed in The United StatesDocument8 pagesCEMENT and CONCRETE RESEARCH. Vol. 4, Pp. 69-76, 1974. Pergamon Press, Inc. Printed in The United StatesTarek ChikerNo ratings yet
- Preventative Maintenance ChecklistDocument1 pagePreventative Maintenance Checklistajaysharma19686191No ratings yet
- Saturated Versus Unsaturated Hydrocarbons C11-5-10Document10 pagesSaturated Versus Unsaturated Hydrocarbons C11-5-10ALongNo ratings yet
- Victoria Brown Coal PropertiesDocument401 pagesVictoria Brown Coal PropertiesShahabuddin SuzanNo ratings yet
- Q1 Weeks-5-6 Module-5 Conchem SSElectiveDocument23 pagesQ1 Weeks-5-6 Module-5 Conchem SSElectiveGeorge EncaboNo ratings yet
- Real Food Market & DeliDocument1 pageReal Food Market & DeliAmplified LocalNo ratings yet
- Ubc - 1974 - A6 - 7 K48Document73 pagesUbc - 1974 - A6 - 7 K48HƯƠNG NGUYỄN LÊ NGỌCNo ratings yet
- XXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973From EverandXXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973No ratings yet
- Chemical plant TaxonomyFrom EverandChemical plant TaxonomyT SwainNo ratings yet
- AgincourtDocument7 pagesAgincourtb52352986No ratings yet
- A Country With Low Rate of Population Growth or Population DeclineDocument4 pagesA Country With Low Rate of Population Growth or Population Declineb52352986No ratings yet
- Physics Kinetmatics NoteDocument2 pagesPhysics Kinetmatics Noteb52352986No ratings yet
- Rest of ChemDocument3 pagesRest of Chemb52352986No ratings yet
- Unit 1 1-8Document7 pagesUnit 1 1-8b52352986No ratings yet
- DualGameProject FoP Group05Document22 pagesDualGameProject FoP Group05arafatahmmed51No ratings yet
- Computer Questions O LevelDocument9 pagesComputer Questions O Levellapsa22No ratings yet
- Curing CciDocument9 pagesCuring CcimfdcontractingNo ratings yet
- A20 User Manual 2013-03-22Document0 pagesA20 User Manual 2013-03-22Stefan S KiralyNo ratings yet
- Dynamic Analysis of Towers: Dr. K. Muthumani & Dr. N.GopalakrishnanDocument154 pagesDynamic Analysis of Towers: Dr. K. Muthumani & Dr. N.GopalakrishnanSube OhNo ratings yet
- Astm D 3164 - 03Document4 pagesAstm D 3164 - 03phaindikaNo ratings yet
- TVF Practice Sum - 2Document14 pagesTVF Practice Sum - 2Rashi MehtaNo ratings yet
- CS325: Analysis of Algorithms, Fall 2016 Midterm Solutions: 2 N N I NDocument5 pagesCS325: Analysis of Algorithms, Fall 2016 Midterm Solutions: 2 N N I NUsama HameedNo ratings yet
- Python Course: Session 6b - Regular ExpressionsDocument11 pagesPython Course: Session 6b - Regular ExpressionsDimitris LyberisNo ratings yet
- Solution Fo AssignmetDocument10 pagesSolution Fo AssignmetnaitikkapadiaNo ratings yet
- SulfurDocument8 pagesSulfurAndreeduis RodriguezNo ratings yet
- Schindler R300: High Performance Roller Guide ShoeDocument2 pagesSchindler R300: High Performance Roller Guide ShoeWashington MazziniNo ratings yet
- Speed Control of DC Motor by Using PWM TechniqueDocument14 pagesSpeed Control of DC Motor by Using PWM TechniquePrincy Merin JoseNo ratings yet
- Chapter 8Document268 pagesChapter 8Matthew B. PolingNo ratings yet
- NX Nastran Theoretical ManualDocument625 pagesNX Nastran Theoretical ManualMSC Nastran BeginnerNo ratings yet
- Module 3 StatsDocument17 pagesModule 3 StatsJasmine BalbinNo ratings yet
- Huawei Certification HCNP Lab Guide HCNP-IENP v1.6 PDFDocument164 pagesHuawei Certification HCNP Lab Guide HCNP-IENP v1.6 PDFGustavo BacaláNo ratings yet
- Green University of Bangladesh Department of Computer Science and Engineering (CSE)Document3 pagesGreen University of Bangladesh Department of Computer Science and Engineering (CSE)HimelNo ratings yet
- ch29 of Mikell P Groover: Principles of Modern Manufacturing, 5th EditionDocument2 pagesch29 of Mikell P Groover: Principles of Modern Manufacturing, 5th EditionLizzsel FranchesNo ratings yet
- ZE245E-10 Technical Specification (EN)Document8 pagesZE245E-10 Technical Specification (EN)Дмитрий КрупинNo ratings yet
- Selectable Output Horns, Strobes, and Horn StrobesDocument4 pagesSelectable Output Horns, Strobes, and Horn StrobesSupriyadi JasminNo ratings yet
- EXERCICESDocument14 pagesEXERCICESabdoul750% (2)
- Normal Distirbution: 5.1 Meaning of Normal DistributionDocument13 pagesNormal Distirbution: 5.1 Meaning of Normal DistributionAllina PonganNo ratings yet
- Minor Project: Object Recognition SystemDocument20 pagesMinor Project: Object Recognition SystemAditya AnandNo ratings yet
- XMT-DS-01661-EN - Impeller 340 BNMB BTU Energy Transmitter Product Data SheetDocument2 pagesXMT-DS-01661-EN - Impeller 340 BNMB BTU Energy Transmitter Product Data SheetorodriguezNo ratings yet
- How To Use VLOOKUP in Excel - A Simple Tutorial Part IDocument28 pagesHow To Use VLOOKUP in Excel - A Simple Tutorial Part IhemanvshahNo ratings yet
- under NDA: 泰凌 Kite BLE SDK 开发指南Document317 pagesunder NDA: 泰凌 Kite BLE SDK 开发指南毛增No ratings yet
- Pratyush Kumar: B.Tech, Engineering Physics, IIT DelhiDocument2 pagesPratyush Kumar: B.Tech, Engineering Physics, IIT DelhiChristopher LindsayNo ratings yet