Professional Documents
Culture Documents
12 Drug Profile and Literature Survey
12 Drug Profile and Literature Survey
Uploaded by
varsha02jadhavOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
12 Drug Profile and Literature Survey
12 Drug Profile and Literature Survey
Uploaded by
varsha02jadhavCopyright:
Available Formats
CHAPTER 1 INTRODUCTION AND OBJECTIVES
1.10 Drug Profile
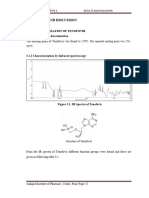
Figure 1.5 Structure of Nevirapine
1.10.1Nevira
pine
1) Chemical name: 11- Cyclopropyl-4-methyl-5, 11-dihydro-6H-dipyride [3,2-
b:2’,3’-e] [1,4] diazepin-6-one.
2) Molecular formula: C15H14N4O
3) Molecular weight: 266.88 g/mol
4) Category: Non –Nucleoside Reverse transcriptase Inhibitor
(NNRTI) belongs to anti-retroviral class
5) Dosage forms: Tablet (Viramune, Boehringer Ingelheim)
6) Appearance: White, amorphous powder.
7) Melting point: 243.06 0C
8) pKa value : 5.06
9) Solubility: Slightly soluble in water, propanol & freely soluble
in methanol and Acetonitrile.
Sinhagad Institute of Pharmacy Narhe Pune - 41 25
CHAPTER 1 INTRODUCTION AND OBJECTIVES
11) Pharmacodynamic profile
Nevirapine selectively inhibit HIV-1 (but not HIV – 2) reverse transcriptase (RT) in a
non-competitive manner.
At 84 nmol/L nevirapine inhibits 50% of HIV-1 RT activity in vitro.
Nevirapine binds preferentially to the p66 subunit of RT at a hydrophobic peptide region
between tyrosine 181 (Tyr-181) and Tyrosine 188 (tyr-188). The binding site is distinctly
separate from the catalytic site of RT. Therefore, substrate binding is unaffected.
Nevirapine inhibits RT by slowing the rate of a magnesium ion dependent chemical
reaction catalyzed by the enzyme . Specifically, Nevirapine binding is thought to alter
the position of the aspartate Carboxyl ligands, which results in the slowing of the
catalytic reaction rate, but it has no effect on nucleotide binding or nucleotide – induced
conformational change.
Nevirapine produces a direct stimulatory effect on HIV RT ribonuclease (RNase H)
activity and alter the cleavage specificity of RNase H. The alternation in RNase H
specificity facilitate multiple cleavage instead of the usual primary cleavage at 18 th
nucleotide from the DNA 3’Terminus.
Nevirapine inhibits HIV-1 RT RNA and DNA dependent DNA polymerase activities in a
template dependent manner, incorporation of deoxyguanosine monophosphate is
preferentially inhibited in the RNA-dependent process versus deoxy thymidine
monophosphate in the DNA- dependent process.
12) Pharmacokinetic properties:
a) Absorption and distribution: NVP is small lipophillic molecule that is rapidly
and almost completely absorbed after oral administration in human .
Maximum plasma concentration achieved at about 4 h after drug administration
and the absolute bioavailability is 93% for the tablet formulation. Ingestion of
nevirapine with food delays the absorption but doesnot affect the bioavailability.
NVP can thus be taken with or without food. It is extensively distributed in the
body with cerebralspinal fluid (CSF) and semen, having concentration of about
30% and 60% respectively.
Sinhagad Institute of Pharmacy Narhe Pune - 41 26
CHAPTER 1 INTRODUCTION AND OBJECTIVES
b) Metabolism and excretion:
The drugs 17 beta-chloromethyl ester function is hydrolyzed to an inactive
carboxylic acid moiety.
13) Mechanism of action:
NNRTI binds allosterically at a distinct site away from the active site termed the
NNRTI pocket.As all NNRTI bind with in the same pocket, viral strain which are
resistant to nevirapine are usually also resistant to other NNRTI’s efavirenz and
delavirdine however second generation NNRTI’s like relpivirdine and etravirine are
effective in treatment for HIV Strain resistant to nevirapine and other first generation
drug in that same class.
14)Adverse effects35:
The most common adverse effect of Nevirapine is the development of mild or
moderate rash (13%) and severe orlife threatening skin reaction have been observed
like Stefenjohnson syndrome, toxic epidermal necrolysis and hypersensitivity (1.5%)
15) Drug interaction:
NVP interact with anti tuberculosis drug like rifampicin, that results in
decrease of its level in blood. Interaction with antiretroviral drug like
Efavirenz, Indinavir, Lopinavir, Nelfinavir, Saquinavir and antifungal drugs
like Clarithromycin , Ketoconazole, NVP reduces the level of these drugs
during co-administration.
NVP decreases anticoagulant effect of Acenocoumaraol, Dicumarol and
Anisindione. As NVP is strong CYP3A4 inducer, it decreases the effect of
Atazanavir, Ketoconazole, Estradiol, Atorvastatin,Roflumilast, Simvastatin,
Lovastatin by increasing their metabolism.It also increases the risk of toxicity
when taken in combination with Quinupristine
Sinhagad Institute of Pharmacy Narhe Pune - 41 27
CHAPTER 1 INTRODUCTION AND OBJECTIVES
1.11 Literature survey
Table 1.9: Literature Survey chart
Sr. YEAR AUTHORS TITLE DETAILS
No
1. 2014 Sichilongo.K, Comparative Chromatography Comparison of GC-MS ,LC-UV in
Chingyana.C, MS Studies on Nevirapine determination of NVP in human
Massele.A. . plasma
2. 2013 Valuru.R, Phani. LC-MS/MS for simultaneous C18 column equipped with a 2.1 ×
B, Reddy.B, determination of Ziduvidine, 10 mm guard column.0.1% Formic
Bagul.R. Lamivudine, Nevirapine in Human acid in Water:Methanol (15:85 v/v)
plasma isocratic. pH 6.3
3. 2013 Zoubir.D, Validation of fast method for C-18 kromasil column ,
Cathenne.F, quantitative analysis of 16 drug Phosphate:Acetonitrile (60:40 v/v)
Claire.T et.al including Nevirapine by UPLC-MS/MS pH 4.6
4. 2013 Valluro.R , LC-MS/MS for simultaneous C-18 AQ Column , 1mM
Kilaru.N. determination of tenofovir, Ammonium Acetate :Acetonitrile
lamivudine,Nevirapine in human plasma (50:50 v/v)
pH 6.5
5. 2012 Umbelina.C, Evidence for Nevirapine bioactivation in First step in mechanism of
Alexandra M. man Nevirapine toxicity.
6. 2012 Murali.K, Simultaneous quantitation of C-18 column
Nageswara.M, lamivudine,ziduvidine, nevirapine in Acetonitrile:Formic acid
Jaswanth.K. human plasma by LC-MS and (76:24 v/v), pH 5.8
application to pharmacokinetic Flow rate 0.8ml/min
7. 2009 Prasada C, Development and validation of RP- C-18 column, Methanol: Acetate
Channabvaraj.C, HPLC method for estimation of buffer (60:40 v/v), pH = 3, flow
Aswini.G. nevirapine in bulk drug and tablet rate 1ml/min ,Wavelength =280nm
8. 2009 Nageswaranrao.R, 2D LC-MS/MS determination of C-18 column for separation by LC
Dhananjay.K, antiretroviral drug in rat serum and urine
Shinde,D.
9. 2009 Fengue.Q, Identification of a process impurity Impurities investigated by series of
Pennino.S, formed during synthesis of Nevirapine photo and oxidative studies
Bussaco.A. Analogue HIV NNRT Inhibitor using 0.1% formic acid and acetonitrile
LC/MS
10. 2007 Thomas.R. Quantitation of five nevirapine oxidative C-18 column 0.8 % Formic acid in
Gregor.T, metabolite in human plasma using LC- Water : Methanol (45:55 v/v)
Campbell.S, et.al MS
11. 2007 Tarinas.A, Bioequivalence study of two nevirapine Dose 200mg
Tapanes.R, tablet in HIV-Infected patient AUC 0.86-1.17
Gorizalez.D. Tmax -2.64
(duration of 12hr)
Sinhagad Institute of Pharmacy Narhe Pune - 41 28
CHAPTER 1 INTRODUCTION AND OBJECTIVES
12. 2007 Mistri.N, High throughput LC-MS Simultaneous C-18 column
Jangid.A, estimation of Lamivudine , 0.5% glacial Acetic acid in water:
Pudage.A et.al Stavudine,Nevirapine in Human plasma Acetonitrile
(20:80 v/v)
13. 2006 Sarkar.M, Development and validation of RP- C-18 column
Khaisdavilli.R HPLC and UV method of analysis for Wavelength =313nm .
Parichagnala.R, quantitative estimation of antiretroviral 0.1% Formic acid in water:
et.al drug in pharmaceutical dosage forms Methanol (50:50 v/v)
14. 2003 Kaul.N, HPTLC Method for determination of Mobile phase
Agarwal.H, Nevirapine in Pharmaceutical dosage TouleneCarbon :
Paradkar.A. form Tetrachloride:Methanol:acetone:am
monia (3.5:3.5:2:1:0.05 % v/v)
Wavelength =289nm
15. 2003 Jingduanchi A, LC-MS/MS method for determination of Mobile phase
Judith.A, Nevirapine in human plasma Buffer:Acetonitrile (80:20% v/v)
Francesca.T. pH = 4.1
m/z of Nevirapine 267/226 628/421
for internal std
16. 2002 Marchei.E, RP-LC determination of Ziduvidine and C-18 column
Valvo.L, Pacifi.R. Nevirapine in Human Plasma Mobile phase
Pot. Dihydrogen phosphate and
acetonitrile (83:17v/v) pH 6.5
wavelength =265 nm
Sinhagad Institute of Pharmacy Narhe Pune - 41 29
You might also like
- Unit Operations in Chemical Industries: November 2019Document20 pagesUnit Operations in Chemical Industries: November 2019Jhon NeverNo ratings yet
- Antiespumantes AshlandDocument28 pagesAntiespumantes AshlandEmilio TafurNo ratings yet
- Astm E1252-2021Document13 pagesAstm E1252-2021Anonymous tIwg2AyNo ratings yet
- REVIEW QUESTIONS CDI 6 (Mariz)Document5 pagesREVIEW QUESTIONS CDI 6 (Mariz)Belinda ViernesNo ratings yet
- 110 WS Gas StoichiometryDocument2 pages110 WS Gas StoichiometryAnsel SotnasNo ratings yet
- Project Report On Stone Paper Manufacturing Cap: 1400 Ton/yearDocument7 pagesProject Report On Stone Paper Manufacturing Cap: 1400 Ton/yearEIRI Board of Consultants and Publishers100% (2)
- jESE Vol2 No2 p67-75 2012Document9 pagesjESE Vol2 No2 p67-75 2012Zulva Chairunnisa BudimanNo ratings yet
- Brief Resume of Intended Work: 6.1: Need For The Study:: Rapid, EconomicalDocument8 pagesBrief Resume of Intended Work: 6.1: Need For The Study:: Rapid, EconomicalDrGajanan VaishnavNo ratings yet
- Cao2008 Article AMethodForQuantifyingTheUnstabDocument9 pagesCao2008 Article AMethodForQuantifyingTheUnstabUsman ArshadNo ratings yet
- Research PratikshaDocument8 pagesResearch PratikshaNutan Desai RaoNo ratings yet
- Fawzy1988 PDFDocument7 pagesFawzy1988 PDFAnonymous MmTwuOanNo ratings yet
- Pre-Column Derivatization Method For DeterminingDocument7 pagesPre-Column Derivatization Method For DeterminingKuanNo ratings yet
- Sensitive Voltammetric Detection of Yeast RNA Based On Its Interaction With Victoria Blue BDocument10 pagesSensitive Voltammetric Detection of Yeast RNA Based On Its Interaction With Victoria Blue Bkontiki500No ratings yet
- Inh y Acihn 2007Document9 pagesInh y Acihn 2007liz zuñigaNo ratings yet
- Rivaroxaban Sensitivity ThromboplastinDocument5 pagesRivaroxaban Sensitivity ThromboplastinpgdlilleNo ratings yet
- Polarographic Analysis of Quetiapine in Pharmaceuticals: Nahed El-Enany, Amina El-Brashy, Fathalla Belal, Nihal El-BahayDocument13 pagesPolarographic Analysis of Quetiapine in Pharmaceuticals: Nahed El-Enany, Amina El-Brashy, Fathalla Belal, Nihal El-BahayPuji LestariNo ratings yet
- Journal of Chromatography BDocument7 pagesJournal of Chromatography BEduardoNo ratings yet
- 814 1584 1 PBDocument11 pages814 1584 1 PBLilia AndromedaNo ratings yet
- Refinement of A Radioreceptor Binding Assay For Nicotinic Acid Adenine Dinucleotide PhosphateDocument11 pagesRefinement of A Radioreceptor Binding Assay For Nicotinic Acid Adenine Dinucleotide PhosphateYsabel Huaccallo AguilarNo ratings yet
- Indian Journal-Rita Lopi FinalDocument5 pagesIndian Journal-Rita Lopi FinalRaja AbhilashNo ratings yet
- STABILITY INDICATING ASSAY METHOD DEVELOPMENT AND VALIDATION OF PREGABALIN IN PHARMACEUTICAL DOSAGE FORMS BY RP-HPLC P.Sneha, Prathima SrinivasDocument10 pagesSTABILITY INDICATING ASSAY METHOD DEVELOPMENT AND VALIDATION OF PREGABALIN IN PHARMACEUTICAL DOSAGE FORMS BY RP-HPLC P.Sneha, Prathima SrinivasiajpsNo ratings yet
- Development and Validation of An RP-HPLC Method For The Determination of Rifapentine in Bulk and Pharmaceutical Dosage FormDocument15 pagesDevelopment and Validation of An RP-HPLC Method For The Determination of Rifapentine in Bulk and Pharmaceutical Dosage Formraju kundavaramNo ratings yet
- 1 s2.0 S2095177921000101 MainDocument10 pages1 s2.0 S2095177921000101 MainLeo EspositoNo ratings yet
- Metodo HPLC FenolDocument7 pagesMetodo HPLC FenolClaudia DiazNo ratings yet
- Simultaneous HPLC Determination of Isoniazid and Acetylisoniazid in PlasmaDocument9 pagesSimultaneous HPLC Determination of Isoniazid and Acetylisoniazid in Plasmarolffspindola4914No ratings yet
- Development of HPLC Method For The Determination of Zinc Carnosine in Bulk and Dosage FormsDocument5 pagesDevelopment of HPLC Method For The Determination of Zinc Carnosine in Bulk and Dosage FormsSouheila MniNo ratings yet
- 330 Simultaneous Determination of Lamivudine Zidovudine and Nevirapine in Tablet Dosage Forms by RP HPLC MethodDocument6 pages330 Simultaneous Determination of Lamivudine Zidovudine and Nevirapine in Tablet Dosage Forms by RP HPLC MethodIka PramithaNo ratings yet
- 1 FDocument11 pages1 FSherlyy Kristiani.SNo ratings yet
- PS03025Document9 pagesPS03025ahmed.bouchenakNo ratings yet
- 10 Drug Profile and Literature SurveyDocument6 pages10 Drug Profile and Literature Surveyvarsha02jadhavNo ratings yet
- Cloning, Expression, and Renaturation Studies of Reteplase: Zhao, Youchun, Wang Ge, Yang Kong, Changkai ZhangDocument4 pagesCloning, Expression, and Renaturation Studies of Reteplase: Zhao, Youchun, Wang Ge, Yang Kong, Changkai ZhangAnindya Rahma O KNo ratings yet
- Nitrosaminas WatersDocument19 pagesNitrosaminas WatersCesar GonzalezNo ratings yet
- Evaluation of acute toxicity of β-lapachone associated with chitosan as a cytoprotective agentDocument9 pagesEvaluation of acute toxicity of β-lapachone associated with chitosan as a cytoprotective agentMatheus MenezesNo ratings yet
- T. Galeano Diaz, M. I. Acedo Valenzuela, F. Salinas: © Springer-Verlag 1994Document10 pagesT. Galeano Diaz, M. I. Acedo Valenzuela, F. Salinas: © Springer-Verlag 199414. Nhật DuyNo ratings yet
- Aac 37 2 178Document5 pagesAac 37 2 178Marcelo salvador silva MacotoraNo ratings yet
- Novel HPLC-UV Method For Simultaneous Determination of Fat-Soluble Vitamins and Coenzyme Q10 in Medicines and SupplementsDocument7 pagesNovel HPLC-UV Method For Simultaneous Determination of Fat-Soluble Vitamins and Coenzyme Q10 in Medicines and Supplementsade muchlasNo ratings yet
- Determination of Diclofenac Sodium and Papaverine Hydrochloride in Tablets by HPLC MethodDocument6 pagesDetermination of Diclofenac Sodium and Papaverine Hydrochloride in Tablets by HPLC MethodDanilo RodriguesNo ratings yet
- Jurnal PenuntunDocument11 pagesJurnal PenuntunAgustinus PotterNo ratings yet
- New Fluorogenic Substrates For Measurement of Angiotensin Converting Enzyme ActivityDocument3 pagesNew Fluorogenic Substrates For Measurement of Angiotensin Converting Enzyme ActivityDani VankovaNo ratings yet
- Synthesis Characterization and Determination of MeDocument6 pagesSynthesis Characterization and Determination of MeKhairiyatul YasminNo ratings yet
- Development and Validation of Stability Indicating Method For The Simultaneous Estimation of Saxagliptin Hydrochloride and Dapagliflozin Using RPHPLC Method in Tablet Dosage FormDocument15 pagesDevelopment and Validation of Stability Indicating Method For The Simultaneous Estimation of Saxagliptin Hydrochloride and Dapagliflozin Using RPHPLC Method in Tablet Dosage FormAdvaita PatelNo ratings yet
- SYNTHESIS, PROAPOPTOTIC ACTIVITY AND 2D-QSAR STUDIES of Some Phenothiazine AnaloguesDocument10 pagesSYNTHESIS, PROAPOPTOTIC ACTIVITY AND 2D-QSAR STUDIES of Some Phenothiazine AnaloguesPeter ZubáčNo ratings yet
- Acid 4Document9 pagesAcid 4LeTienDungNo ratings yet
- Journal of Chromatography B: Huaibing He, Ester Carballo-Jane, Xinchun Tong, Lucinda H. CohenDocument8 pagesJournal of Chromatography B: Huaibing He, Ester Carballo-Jane, Xinchun Tong, Lucinda H. CohenKuanNo ratings yet
- Ijbms 18 979Document10 pagesIjbms 18 979jlizpinedaNo ratings yet
- Fourdrugspaper - Domperidone Stress Degradation StudyDocument17 pagesFourdrugspaper - Domperidone Stress Degradation Studyanggi yudhatamaNo ratings yet
- Article WJPR 1408528531Document9 pagesArticle WJPR 1408528531sripathy84No ratings yet
- Acetyl Cysteine Literature Corrections MadeDocument22 pagesAcetyl Cysteine Literature Corrections MademagimaryNo ratings yet
- Conversion Factor ReninDocument7 pagesConversion Factor RenindasdNo ratings yet
- Tuberculosis With Discordant Drug Resistance Patterns - A Diagnostic DilemmaDocument4 pagesTuberculosis With Discordant Drug Resistance Patterns - A Diagnostic DilemmaKevinNo ratings yet
- Stability Indicating RP-HPLC Method For The Estimation of Racecadotril in Pharmaceutical Dosage FormDocument8 pagesStability Indicating RP-HPLC Method For The Estimation of Racecadotril in Pharmaceutical Dosage FormAnjay MalikNo ratings yet
- Nama: Hasby Zaen Attoillah NIM: 1012016034 Summary ofDocument2 pagesNama: Hasby Zaen Attoillah NIM: 1012016034 Summary ofFuri TriyasNo ratings yet
- Validated RP-HPLC Method For The Determination of Nelaribine in Bulk and Tablet Dosage FormDocument10 pagesValidated RP-HPLC Method For The Determination of Nelaribine in Bulk and Tablet Dosage FormEditor IJTSRDNo ratings yet
- Niacinamide JurnalDocument8 pagesNiacinamide JurnalAnnisaaNo ratings yet
- Simultaneous Determination of Glipizide and Glimepride by RP-HPLC in Dosage Formulations and in Human SerumDocument6 pagesSimultaneous Determination of Glipizide and Glimepride by RP-HPLC in Dosage Formulations and in Human SerumAshique RajputNo ratings yet
- en 1690228457191Document12 pagesen 1690228457191Angel GarciaNo ratings yet
- Hypertensionaha 119 12703Document9 pagesHypertensionaha 119 12703Hector PonsNo ratings yet
- Cloud-Point Extraction For The Determination of The Free Fraction of Antiepileptic Drugs in Blood Plasma and SalivaDocument5 pagesCloud-Point Extraction For The Determination of The Free Fraction of Antiepileptic Drugs in Blood Plasma and SalivaDan AndonieNo ratings yet
- The in Vitro Effects of Ranitidine Hydrochloride On The Protein Binding of Naproxen Sodium in Aqueous MediumDocument7 pagesThe in Vitro Effects of Ranitidine Hydrochloride On The Protein Binding of Naproxen Sodium in Aqueous MediumIrfan N KhanNo ratings yet
- CCLM 46 8 1127 PDFDocument8 pagesCCLM 46 8 1127 PDFJayaNo ratings yet
- Piao 2008Document5 pagesPiao 2008Ellie satrianiNo ratings yet
- Valporate 3Document7 pagesValporate 3sisnaingaungNo ratings yet
- In-Vitro Cellular Uptake and Transport Study of 9-NitrocamptothecinDocument10 pagesIn-Vitro Cellular Uptake and Transport Study of 9-NitrocamptothecinMonica TurnerNo ratings yet
- Article Wjpps 1425131684Document16 pagesArticle Wjpps 1425131684Azlin ApriantoNo ratings yet
- Ginseng Powders by Ultra Performance Liquid ChromatographyDocument10 pagesGinseng Powders by Ultra Performance Liquid ChromatographyPopescu BiancaNo ratings yet
- Analytical Methods for Major and Modified Nucleosides - HPLC, GC, MS, NMR, UV and FT-IRFrom EverandAnalytical Methods for Major and Modified Nucleosides - HPLC, GC, MS, NMR, UV and FT-IRNo ratings yet
- Sap CVDocument2 pagesSap CVvarsha02jadhavNo ratings yet
- FouadspaperinIJCS2014Document12 pagesFouadspaperinIJCS2014varsha02jadhavNo ratings yet
- Oxid 1Document2 pagesOxid 1varsha02jadhavNo ratings yet
- Mental Health Challenges in YouthDocument8 pagesMental Health Challenges in Youthvarsha02jadhavNo ratings yet
- Oxid 3Document2 pagesOxid 3varsha02jadhavNo ratings yet
- Procure To Pay Cycle - CERTIFICATION.1Document9 pagesProcure To Pay Cycle - CERTIFICATION.1varsha02jadhavNo ratings yet
- Oxid 5 MinDocument2 pagesOxid 5 Minvarsha02jadhavNo ratings yet
- AnalyticalOutsourcingDocument6 pagesAnalyticalOutsourcingvarsha02jadhavNo ratings yet
- FormatDocument2 pagesFormatvarsha02jadhavNo ratings yet
- 17 Resultant DiscussionDocument35 pages17 Resultant Discussionvarsha02jadhavNo ratings yet
- Oxid 2Document2 pagesOxid 2varsha02jadhavNo ratings yet
- 00 Cover Page For Golden EmbossingDocument1 page00 Cover Page For Golden Embossingvarsha02jadhavNo ratings yet
- 03 AcknowledementDocument2 pages03 Acknowledementvarsha02jadhavNo ratings yet
- 16 MethodsDocument9 pages16 Methodsvarsha02jadhavNo ratings yet
- 15 MATERIAL AND METHODS NewDocument1 page15 MATERIAL AND METHODS Newvarsha02jadhavNo ratings yet
- 10 Drug Profile and Literature SurveyDocument6 pages10 Drug Profile and Literature Surveyvarsha02jadhavNo ratings yet
- 02 Certificates DinalDocument2 pages02 Certificates Dinalvarsha02jadhavNo ratings yet
- 19 ReferencesDocument4 pages19 Referencesvarsha02jadhavNo ratings yet
- 12chapter 3 Results and DiscussionDocument33 pages12chapter 3 Results and Discussionvarsha02jadhavNo ratings yet
- FigureDocument2 pagesFigurevarsha02jadhavNo ratings yet
- Ultracrete PC730Document3 pagesUltracrete PC730uchennaNo ratings yet
- Structure Elucidation: Fonnallon An Oxime ofDocument5 pagesStructure Elucidation: Fonnallon An Oxime ofHina AftabNo ratings yet
- Carbohydrate Polymers: Jie Ren, Hongye Fu, Tianbin Ren, Weizhong YuanDocument7 pagesCarbohydrate Polymers: Jie Ren, Hongye Fu, Tianbin Ren, Weizhong YuanSiddharthBhasneyNo ratings yet
- Belviso - 2018 - State-Of-The-Art Applications of Fly Ash From Coal and Biomass A Focus On Zeolite Synthesis Processes and Issues-AnnotatedDocument27 pagesBelviso - 2018 - State-Of-The-Art Applications of Fly Ash From Coal and Biomass A Focus On Zeolite Synthesis Processes and Issues-AnnotatedNikosNo ratings yet
- Electrochemical Preparation of CuBi2O4 Nanoparticles On Nanoporous Stainless Steel As A Binder-Free Supercapacitor ElectrodeDocument9 pagesElectrochemical Preparation of CuBi2O4 Nanoparticles On Nanoporous Stainless Steel As A Binder-Free Supercapacitor ElectrodemuhammadNo ratings yet
- Uissolq) Ffi Tfiff: .'Tfiii' ItDocument13 pagesUissolq) Ffi Tfiff: .'Tfiii' ItMUHAMMAD YASEENNo ratings yet
- Zytel® 45HSB NC010-gbDocument14 pagesZytel® 45HSB NC010-gbJuan Fernando CampuzanoNo ratings yet
- PreviewpdfDocument102 pagesPreviewpdfGowrishankarNo ratings yet
- 19 Volumetric Analysis Involving Acids and Alkalis Teacher PDFDocument63 pages19 Volumetric Analysis Involving Acids and Alkalis Teacher PDFVanessa LeungNo ratings yet
- Separating TechniquesDocument13 pagesSeparating TechniquesAnalysa SinghNo ratings yet
- WEEK 3 ActivityDocument11 pagesWEEK 3 ActivityMai SasaNo ratings yet
- Food and Beverage Can Coatings A Review On Chemicalanalysis, Migration, and Risk AssessmentDocument54 pagesFood and Beverage Can Coatings A Review On Chemicalanalysis, Migration, and Risk AssessmentAna Julia Mayumi PupinNo ratings yet
- Continuous Waste Tire AnalysisDocument49 pagesContinuous Waste Tire Analysis202040336No ratings yet
- Concept Strengthening Sheet (CSS-05) Based On AIATS-05 (CF+OYM) - ChemistryDocument4 pagesConcept Strengthening Sheet (CSS-05) Based On AIATS-05 (CF+OYM) - Chemistryshakuntla6413No ratings yet
- Understanding ElectrochemistryDocument3 pagesUnderstanding ElectrochemistryMarisol GómezNo ratings yet
- Technology Sheet Degassing of Liqids Using Membrane TechnologyDocument6 pagesTechnology Sheet Degassing of Liqids Using Membrane TechnologysalcedopozasNo ratings yet
- Poster Template - 2Document1 pagePoster Template - 2Mai EkafNo ratings yet
- Kelly 1999Document6 pagesKelly 1999karenNo ratings yet
- Unit-4 NanotechnologyDocument18 pagesUnit-4 NanotechnologyZayeem Zehek67% (3)
- D.T.D.900AA: Ministry of Defence Defence Procurement Agency, ADRP2 Abbey Wood Bristol BS34 8JHDocument24 pagesD.T.D.900AA: Ministry of Defence Defence Procurement Agency, ADRP2 Abbey Wood Bristol BS34 8JHSatyendra PandeyNo ratings yet
- Periodic Classification PYQsDocument31 pagesPeriodic Classification PYQsa9758127118No ratings yet
- DocumentDocument21 pagesDocumentcᴘcтԍᴀмιɴԍ YTNo ratings yet
- Annotated Vcaa Chemistry Data BookletDocument12 pagesAnnotated Vcaa Chemistry Data Booklettkn5520No ratings yet
- Thermoplastic Foaming Mechanism and PB Adsorption of Poly (Vinyl Alcohol) /shell Powder Porous CompositeDocument11 pagesThermoplastic Foaming Mechanism and PB Adsorption of Poly (Vinyl Alcohol) /shell Powder Porous CompositeWq ZNo ratings yet