Professional Documents
Culture Documents
Agent Used in Disorder in Coagulation
Uploaded by
Clinton Franda Markus Sitanggang0 ratings0% found this document useful (0 votes)
27 views34 pagesOriginal Title
agent used in disorder in coagulation.pptx
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
27 views34 pagesAgent Used in Disorder in Coagulation
Uploaded by
Clinton Franda Markus SitanggangCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You are on page 1of 34
Drug Used in Disorder of Coagulation
(Including drug used to limit abnormal bleeding and to inhibit
thrombosis)
ANGGELIA P, MD
PHARMACOLOGY AND THERAPEUTIC DEPT.
FACULTY OF MEDICINE
UNIVERSITY OF JAMBI, INDONESIA
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 1
NORMAL HEMOSTATIC MECHANISM
Hemostasis refers to the finely regulated dynamic
process of maintaining fluidity of the blood,
repairing vascular injury, and limiting blood loss
while avoiding vessel occlusion (thrombosis) and
inadequate perfusion of vital organs.
There are four phases to the
normal coagulation cascade:
Blood vessel constriction
Platelet aggregation
Fibrin generation
Vessel repair and fibrin
degradation
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 2
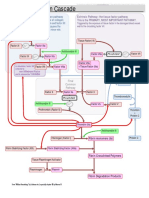
MECHANISM OF BLOOD COAGULATION
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 3
PLATELET PLUG
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 4
COAGULATION CASCADE
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 5
COAGULATION CASCADE
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 6
INHIBITION OF COAGULATION
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 7
INTRODUCTION
Human body controlling coagulation process by activated plasmin, antithrombine
synthesized and activating protein C and S.
Antitrombotic:
I. anticoagulation
• Indirect thrombin inhibitor (heparin : UFH, LMWH, synthetic heparin
derivates…fondaparinux)
• Direct trombin inhibitor (hirudin_lepirudine, bivalirudin, argatroban, melagatran)
• Vit K antagonist (warfarin and coumarin derivates)
• Direct Xa inhibitor (rivaroxaban)
II. Fibrinolytic drug (streptokinase, urokinase, t-Pas_alteplase, reteplase, teneplase)
III. Antiplatelet drug (aspirin, clopidrogel, abciximab, dypiridamol+aspirin, cilostazol)
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 8
ANTICOAGULANT
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 9
ANTICOAGULANT DRUGS
Vitamin K Direct thrombin
Heparins antagonists inhibitors
Unfractionated Warfarin Hirudin
heparin (UFH)
Coumarin Recombinant
Low molecular - Lepirudin
weight heparin - Bivalirudin
(LMWH)
Synthetic
Synthetic - Argatroban
pentasaccharides
- Melagatran
(fondaparinux,
- Dabigatran
idraparinux)
-
ANTICOAGULANT
I. INDIRECT TROMBIN INHIBITOR
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 12
INDIRECT THROMBIN INHIBITOR (HEPARIN)
Heparin is a heterogeneous mixture of sulfated
mucopolysaccharides
Mechanism of action
Biologic activity is dependent upon the endogenous
anticoagulant antithrombin.
Cofactor that accelerated by 1000-fold antithrombin inhibits
clotting factor proteases, especially thrombin (IIa), IXa, and Xa,
by forming equimolar stable complexes with them.
Heparin extract from porcine intestinal mucosa or bovine lung
Antidotum: protamine sulfate
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 13
INDIRECT THROMBIN INHIBITOR (HEPARIN)
Consist of:
Unfractionated heparin (height weight moleculer heparin)_UFH
Low weight moleculer heparin (enoxaparin, dalteparin, and
tinzaparin) _LMH
Synthesized derivated heparin (Fondaparinux ).
LMH inhibit activated factor X but have less effect on thrombin
than the HMW species
LMH have equal efficacy with UHF, increased bioavailability by
SC inj and less frequent doses.
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 14
INDIRECT THROMBIN INHIBITOR (HEPARIN)
Routine monitoring aPTT need for used of UHF, just adjusment
needed. Maintain prolongation of the aPTT to 2–2.5 times
Coagulation monitoring for LMH used is not done routinely, LMH
giving once/twice/day with fixed dose or /kgbb
Fondaparinux can be given once a day at a fixed dose without
coagulation monitoring.
Precaution for renal failure patient.
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 15
INDIRECT THROMBIN INHIBITOR (TOXICITY)
Bleeding (Increased risk in elder women and renal failure
Allergy reaction
Alopesia or increased loss of hair
Long term therapy: osteoporosis, spontaneous fracture and
deficiency of mineralocorticoid.
Heparin induced trombocitopenia, occurs in 1–4% of individuals
treated with UFH for a minimum of 7 days.
KI: patient with bleeding or risk of bleeding.
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 16
ANTICOAGULANT
II. DIRECT TROMBIN INHIBITOR
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 17
DIRECT THROMBIN INHIBITOR
Mechanism of action by directed binding to the active site of thrombin
Hirudin and bivalirudin are bivalent DTIs in that they bind at both the catalytic or
active site of thrombin as well as at a substrate recognition site.
Argatroban and melagatran are small molecules that bind only at the thrombin
active site.
Hirudin recombinant_lepirudin…. approved by the Food and Drug Administration
for use in patients with thrombosis related to heparin-induced thrombocytopenia.
Parenterally used
Lepirudin is excreted by the kidney and should be used with great caution in
patients with renal insufficiency as no antidote exists
Bivalirudin also inhibits platelet activation and has been FDA-approved for use in
percutaneous coronary angioplasty.
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 18
DIRECT TROMBIN INHIBITOR
Argatroban
• FDA-approved for use in patients with HIT with or without thrombosis and coronary
angioplasty in patients with HIT.
• Has a short half-life, is given by continuous intravenous infusion, and is monitored by
aPTT.
• Its clearance is not affected by renal disease but is dependent on liver function.
Ximelagatran
• Oral prodrug that is metabolized to the DTI melagatran.
• Potential advantages of ximelagatran include predictable pharmacokinetics and
bioavailability.
• This allows for fixed dosing and predictable anticoagulant response; no need for routine
coagulation monitoring; lack of interaction with P450-interacting drugs; and rapid onset
and offset of action,
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 19
DIRECT TROMBIN INHIBITOR
Dabigatran
• Direct competitive inhibition of trombin, inhibit free and clot bound trombin and
trombin induced platelet aggregation
• Not a substrate, inhibitor or inducer of CYP450 enzyme
• T1/2 12-17 h, oral dosing form, BID
• Urine excretion up to 80%
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 20
ANTICOAGULANT
III. VITAMIN K ANTAGONIST
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 21
VITAMIN K ANTAGONISTS – MECHANISM OF
ACTION
VITAMIN K ANTAGONISTS – MECHANISM OF
ACTION
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 23
VITAMIN K-DEPENDENT CLOTTING FACTORS
Varying half-life (6-72 hours)
Anticoagulant effect of vitamin K antagonists starts after
several days
VIT K ANTAGONIST (WARFARIN)
• Warfarin is generally administered as the sodium salt and has 100%
bioavailability.
• Over 99% of racemic warfarin is bound to plasma albumin, which may contribute
to its small volume of distribution (the albumin space), its long half-life in plasma
(36 hours), and the lack of urinary excretion of unchanged drug.
• Warfarin crosses the placenta readily and can cause a hemorrhagic disorder in
the fetus.
• Treatment with warfarin should be initiated with standard doses of 5-10 mg
rather than the large loading doses formerly used.
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 25
WARFARIN
Slow onset and offset of action. This takes about 3-4 days.
The initial treatment with VKAs, such as warfarin, must therefore
always be combined with heparin
When rapid reversal is required, fresh frozen plasma or coagulation
factor concentrates can be administered. Vitamin K can also be
administered but the effect of this treatment is delayed.
Numerous interactions with alcohol, foods containing vitamin K, with
foods and drugs metabolised by cytochrome P450 enzymes and with
foods and drugs which interfere with gastrointestinal absorption
Paper 1; pharmacodynamic and kinetic drug interaction of warfarin
RIVAROXABAN (NEW ORAL ANTICOAGULAN)
• Factor Xa inhibitor that inhibit platelet activation by selectively blocking the active
site of factor Xa without requiring cofactor (eg. Antitrombin) fir activity
• Bioavailability : 80-100% ; Protein 92-95%; metabolized by oxidative degradation
catalyzed by CYP3A4/5; T1/2 5-9 h; excreted in feces and urine.
• PO dosing form
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 27
FIBRINOLYTIC DRUG
Lyse thrombin by catalyzing of plasmin
Consist of: Streptokinase, urokinase, t-Pas(alteplase, reteplase, tenecteplase)
Streptokinase enzym synthesized by streptococci that combines with the
proactivator plasminogen.
Urokinase is a human enzyme synthesized by the kidney that directly converts
plasminogen to active plasmin.
Plasminogen can also be activated endogenously by tissue plasminogen
activators (t-PAs). Human t-PA is manufactured as alteplase by means of
recombinant DNA technology.
Indication : pulmonary embolism with hemodynamic instability, severe deep
venous thrombosis such as the superior vena caval syndrome, and ascending
thrombophlebitis of the iliofemoral vein with severe lower extremity edema.
Golden period, 6 hours after symptom onset of MCI
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 28
ANTIPLATELET DRUG
1. Inhibition of PGI2 (TXA2)….prevent paltelet aggregation…aspirin
2. Inhibition of ADP…no effect of PGI2 (TXA2)….clopidrogel
3. Blockage of platelet glycoprotein II A/III A receptor…abciximab, tirofiban, and
eptifibatide
4. Additional antiplatelet…dypiridamol, cilostazol (phospodiesterase inhibitor ,
promote vasodilatation)
Paper II: Pharmacology antiplatelet drug !!!!
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 29
SYSTEMIC HEMOSTATIC
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 30
PLASMA FRACTION
Cryoprecipitate
FFP
Prothrombin complex concentrates (intermediate purity factor IX concentrates)
Recombinant factor VIII products
Recombinant factor IX products
PAPER 3 clinical used?
+ desmopresisn asetat…synthetic vasopresin…increased Fc VIII and vWf…
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 31
VIT K
Vit K related cascade factor IIa, VII, IX, and X
K1: leafy green vegetables…..orally and parenteral
Onset 6 h…completely action 24 hours, newborn routine
administration to prevent hemorrage.
K2: synt by bacteria in human intestine
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 32
FIBRINOLITIC INHIBITOR
Aminocaproic acid (EACA)….competitively Inhibit plasminogen
activation
Rapidly orally absorbed and excretion via urine.
Adverse effect: skin rash, hipotensi, nose stifness… be aware of
general trombosis
Tranexamid acid (analog aminocaproic acid)…10x more potent
and less side effect. Available in indonesia
Rapidly absorbed, dose 0,5-1 g…2-3x/daily…slowly IV injc…
Th/ for bleeding ec fibrinolitic therapy….avoid used for DIC
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 33
TERIMAKASIH
ANGGELIA P, MD. PHARMACOLOGY AND THERAPEUTIC DEPT. 34
You might also like
- Agent Used in Disorder in CoagulationDocument41 pagesAgent Used in Disorder in CoagulationArtika MayandaNo ratings yet
- Anti CoagulantsDocument37 pagesAnti CoagulantsMaria khurshidNo ratings yet
- ReviewerDocument20 pagesReviewerKC PalattaoNo ratings yet
- Drugs Used To Affect in Blood Coagulation: Prajogo WibowoDocument44 pagesDrugs Used To Affect in Blood Coagulation: Prajogo WibowoIda Bagus Putu SwabawaNo ratings yet
- Presentation. 111tom AbDocument20 pagesPresentation. 111tom AbTemesgenNo ratings yet
- Anticoagulants 161120143945Document29 pagesAnticoagulants 161120143945Roshan SahuNo ratings yet
- Drugs Used in Coagulation Disorders and Agents Used in DyslipidemiaDocument20 pagesDrugs Used in Coagulation Disorders and Agents Used in Dyslipidemiamjd13mjd4No ratings yet
- Anticoagulants May BeDocument5 pagesAnticoagulants May BeAmmar magdyNo ratings yet
- Anticoagulantes ParenteralesDocument19 pagesAnticoagulantes ParenteralesCamilo PérezNo ratings yet
- Receiving Concurrent Moderate CYP3A4 Inhibitors (Erythromycin, Saquinavir, Verapamil, Fluconazole) - 25 MG Once Daily InitiallyDocument272 pagesReceiving Concurrent Moderate CYP3A4 Inhibitors (Erythromycin, Saquinavir, Verapamil, Fluconazole) - 25 MG Once Daily InitiallyFatima Doran PandaogNo ratings yet
- Drugs Used in Disorders of CoagulationDocument60 pagesDrugs Used in Disorders of CoagulationTwinkle MazaredoNo ratings yet
- AnticoagulantDocument34 pagesAnticoagulantAslam Baltee100% (1)
- The Pharmacology of AntithromboticsDocument14 pagesThe Pharmacology of AntithromboticsanaNo ratings yet
- Pharmacology of Anticoagulants: Chandravathi Loke, MD, Syed S. Ali, MD, and Vandita Johari, MDDocument7 pagesPharmacology of Anticoagulants: Chandravathi Loke, MD, Syed S. Ali, MD, and Vandita Johari, MDluqmanhasansNo ratings yet
- Heparin - ClinicalKeyDocument85 pagesHeparin - ClinicalKeydayannaNo ratings yet
- AnticoagulatsDocument52 pagesAnticoagulatsanaya khan StudentNo ratings yet
- Anticoagulant TherapyDocument41 pagesAnticoagulant Therapyryan yovanNo ratings yet
- Mid Human Pharma 23-24Document6 pagesMid Human Pharma 23-24Linh NguyễnNo ratings yet
- AnticoagulantsDocument8 pagesAnticoagulantsRobert L G MabongaNo ratings yet
- Anticoagulants by DR TariqDocument46 pagesAnticoagulants by DR Tariqsinan kNo ratings yet
- AntiarrhythmiaDocument29 pagesAntiarrhythmiaDRx Raju ChandranNo ratings yet
- 12 MalariaDocument61 pages12 MalariaMewael TesfamichaelNo ratings yet
- Anticoagulants: Presented By: ABHILASH Moderator: DR CH RAHUL MDDocument103 pagesAnticoagulants: Presented By: ABHILASH Moderator: DR CH RAHUL MDabhilashreddy45100% (1)
- Chest AC ParenteralesDocument20 pagesChest AC ParenteralescarolinapolotorresNo ratings yet
- Drug StudyDocument5 pagesDrug StudyJoyce Anne SupnetNo ratings yet
- Drugs Used in Disorders of CoagulationDocument61 pagesDrugs Used in Disorders of CoagulationDUEÑAS, MARIELNo ratings yet
- Anticoagulant PresentationDocument29 pagesAnticoagulant Presentationrozha100% (2)
- 3 - Blood MedicationDocument16 pages3 - Blood Medicationcrstian spatariNo ratings yet
- Banua, Latigar, Rona - PCOL 2 - ReviewerDocument61 pagesBanua, Latigar, Rona - PCOL 2 - ReviewerDiane BanuaNo ratings yet
- Anticoagulant, Fibrinolytic, and Antiplatelet DrugsDocument22 pagesAnticoagulant, Fibrinolytic, and Antiplatelet DrugsVera Waty100% (1)
- AnticoagulantsDocument19 pagesAnticoagulantsOsama ZbedaNo ratings yet
- New Oral New Oral Anticoagulants G: RB H TT MD Rebecca Hanratty, MD Denver Health April 12, 2011Document55 pagesNew Oral New Oral Anticoagulants G: RB H TT MD Rebecca Hanratty, MD Denver Health April 12, 2011andresrgomezNo ratings yet
- Tishk International University: ApixabanDocument4 pagesTishk International University: ApixabanDyar MzafarNo ratings yet
- L8 Anti Coagulant DrugsDocument10 pagesL8 Anti Coagulant DrugsamanabduwahabNo ratings yet
- New Drugs 2018 TableDocument5 pagesNew Drugs 2018 TableKirthikaRaghuramanNo ratings yet
- Pharmacology - Hematologic DrugsDocument51 pagesPharmacology - Hematologic DrugsBenjamin Joel Breboneria100% (1)
- Newer Oral Anticoagulant: DR Shivaom Chaurasia Resident Internal MedicineDocument57 pagesNewer Oral Anticoagulant: DR Shivaom Chaurasia Resident Internal MedicineMuhammad Reza FirdausNo ratings yet
- Anticoagulantes ParenteralesDocument23 pagesAnticoagulantes ParenteralesNube AzulNo ratings yet
- DabigatranDocument6 pagesDabigatranNayelis SalasNo ratings yet
- Drugs Use in HemostasisDocument47 pagesDrugs Use in HemostasiskadibhaNo ratings yet
- New Drugs 2018: New Drug Mechanism of Action UseDocument7 pagesNew Drugs 2018: New Drug Mechanism of Action UsePremangshu GhoshalNo ratings yet
- Anticoagulation PharmacologyDocument36 pagesAnticoagulation PharmacologyaymenNo ratings yet
- Myrin P ForteDocument3 pagesMyrin P ForteJohn Zedric Villanueva ArciagaNo ratings yet
- AnticoagulantsDocument64 pagesAnticoagulantsnainapattnaikNo ratings yet
- Bivalirudine Drug Presentation From Nicvd DhakaDocument28 pagesBivalirudine Drug Presentation From Nicvd DhakaNavojit ChowdhuryNo ratings yet
- Heparin ResistanceDocument7 pagesHeparin ResistanceAli JENDOUBINo ratings yet
- BJH Heparin GuidelinesDocument16 pagesBJH Heparin Guidelinesd40sithui100% (1)
- Drug-Laboratory InteractionsDocument3 pagesDrug-Laboratory Interactionsmusic.asia29No ratings yet
- Blood Pharmacology by Dr. Mayur Sayta M 910444Document21 pagesBlood Pharmacology by Dr. Mayur Sayta M 910444funzz100% (1)
- Point of View Heparin ResistanceDocument20 pagesPoint of View Heparin ResistanceSameer Goyal100% (1)
- Blok 5 Hemostatic DrugDocument39 pagesBlok 5 Hemostatic DrugPutri HusnanNo ratings yet
- Anticoagulant: Mechanism and ReverseDocument22 pagesAnticoagulant: Mechanism and ReverseNovi Riyadhah Ma'sumNo ratings yet
- Antiplatelet, Thrombolitik, Antikoagulan, Vasodilator Referat VaskularDocument23 pagesAntiplatelet, Thrombolitik, Antikoagulan, Vasodilator Referat Vaskularmonyet65No ratings yet
- Thrombolytic AgentDocument4 pagesThrombolytic AgentAbdullahIchsanNo ratings yet
- Drugs Affecting Blood Cloting 2019Document39 pagesDrugs Affecting Blood Cloting 2019Mutiara RizkiNo ratings yet
- Newer Oral Anticoagulants: Dabigatran Etexilate Is An Inactive Pro-DrugDocument5 pagesNewer Oral Anticoagulants: Dabigatran Etexilate Is An Inactive Pro-DrugLouiseNo ratings yet
- Obat Sistem HematologiDocument21 pagesObat Sistem HematologiSuryana AdityaNo ratings yet
- Heparin Resistance - Clinical Perspectives and Management StrategiesDocument7 pagesHeparin Resistance - Clinical Perspectives and Management StrategiesRaul DoctoNo ratings yet
- Medicinal Chemistry of Drugs Affecting Cardiovascular and Endocrine SystemsFrom EverandMedicinal Chemistry of Drugs Affecting Cardiovascular and Endocrine SystemsNo ratings yet
- VBNDocument9 pagesVBNClinton Franda Markus SitanggangNo ratings yet
- Visual Acuity Outcomes After CataractDocument10 pagesVisual Acuity Outcomes After CataractClinton Franda Markus SitanggangNo ratings yet
- Global Prevalence of Presbyopia and VisionDocument8 pagesGlobal Prevalence of Presbyopia and VisionClinton Franda Markus SitanggangNo ratings yet
- Injection of Cultured Cells With A ROCKDocument9 pagesInjection of Cultured Cells With A ROCKClinton Franda Markus SitanggangNo ratings yet
- Jurnal MataDocument12 pagesJurnal Matameris dindaNo ratings yet
- Diagnosis and Treatment of Acute RetinalDocument11 pagesDiagnosis and Treatment of Acute RetinalClinton Franda Markus SitanggangNo ratings yet
- Suicide by Ligature Strangulation: Three Case ReportsDocument4 pagesSuicide by Ligature Strangulation: Three Case ReportsAnastasia Eka PuteriNo ratings yet
- 3Document1 page3Clinton Franda Markus SitanggangNo ratings yet
- Suicide Decapitation by A Detonating Cord A Case.9Document4 pagesSuicide Decapitation by A Detonating Cord A Case.9Clinton Franda Markus SitanggangNo ratings yet
- Delayed Homicides and The Proximate Cause: Peter Lin, MD, and James R. Gill, MDDocument4 pagesDelayed Homicides and The Proximate Cause: Peter Lin, MD, and James R. Gill, MDClinton Franda Markus SitanggangNo ratings yet
- Review of A Forensic Pseudoscience Identification of Criminals Frombitemark PatternsDocument6 pagesReview of A Forensic Pseudoscience Identification of Criminals Frombitemark PatternsClinton Franda Markus Sitanggang100% (1)
- Tenofovir Versus Placebo To Prevent PerinatalDocument13 pagesTenofovir Versus Placebo To Prevent PerinatalClinton Franda Markus SitanggangNo ratings yet
- Observations On Increased Accidental Asphyxia.2Document4 pagesObservations On Increased Accidental Asphyxia.2Clinton Franda Markus SitanggangNo ratings yet
- Minimally Invasive Versus Abdominal Radical HysterectomyDocument10 pagesMinimally Invasive Versus Abdominal Radical HysterectomyClinton Franda Markus SitanggangNo ratings yet
- Maintenance Olaparib in Patients With Newly DiagnosedDocument10 pagesMaintenance Olaparib in Patients With Newly DiagnosedClinton Franda Markus SitanggangNo ratings yet
- Neurorehabilitasi Nyeri KronisDocument60 pagesNeurorehabilitasi Nyeri KronisWegrimel AriegaraNo ratings yet
- New England Journal Medicine: The ofDocument10 pagesNew England Journal Medicine: The ofTiffany Rachma PutriNo ratings yet
- Crs RehabDocument8 pagesCrs RehabClinton Franda Markus SitanggangNo ratings yet
- Maintenance Olaparib in Patients With Newly DiagnosedDocument11 pagesMaintenance Olaparib in Patients With Newly DiagnosedClinton Franda Markus SitanggangNo ratings yet
- Discogenic LBP Revised September 2017Document13 pagesDiscogenic LBP Revised September 2017Clinton Franda Markus SitanggangNo ratings yet
- Maintenance Olaparib in Patients With Newly DiagnosedDocument11 pagesMaintenance Olaparib in Patients With Newly DiagnosedClinton Franda Markus SitanggangNo ratings yet
- Crs RehabDocument8 pagesCrs RehabClinton Franda Markus SitanggangNo ratings yet
- 04 - Postterm PregnancyDocument33 pages04 - Postterm PregnancyClinton Franda Markus SitanggangNo ratings yet
- Discogenic Lumbar PainDocument9 pagesDiscogenic Lumbar PainPrimas Shahibba RidhwanaNo ratings yet
- Modalitas RehabmedikDocument102 pagesModalitas RehabmedikClinton Franda Markus SitanggangNo ratings yet
- Scalloped Pupil in A Patient With Familial Amyloid PolyneuropathyDocument2 pagesScalloped Pupil in A Patient With Familial Amyloid PolyneuropathyClinton Franda Markus SitanggangNo ratings yet
- Nervus Cranialis 257Document8 pagesNervus Cranialis 257Clinton Franda Markus SitanggangNo ratings yet
- Aph Obp2 Blok17Document26 pagesAph Obp2 Blok17iqiqiqiqiq100% (1)
- Hasil Analisis Data SPSS Univariat: 1. PerokokDocument5 pagesHasil Analisis Data SPSS Univariat: 1. PerokokClinton Franda Markus SitanggangNo ratings yet
- Clotting Factor Lesson Plan Recovered)Document5 pagesClotting Factor Lesson Plan Recovered)Rebecca Jolie100% (1)
- Anticoagulants and Antiplatelet AgentsDocument4 pagesAnticoagulants and Antiplatelet AgentsMark Russel Sean LealNo ratings yet
- Clinpath-04.-Disorders of Hemostasis and Blood CoagulationDocument11 pagesClinpath-04.-Disorders of Hemostasis and Blood CoagulationCharisse Angelica MacedaNo ratings yet
- 506 Qs Mohammad Mordi @NAJMDocument115 pages506 Qs Mohammad Mordi @NAJMElsayedNo ratings yet
- REVALIDA COMPRE HEMA2LAB MIXING STUDIES UnivSantoTomasDocument4 pagesREVALIDA COMPRE HEMA2LAB MIXING STUDIES UnivSantoTomasJemimah CainoyNo ratings yet
- Assessment of Snake Antivenom Activity of Tamarindus Indica Seed Coat ExtractDocument9 pagesAssessment of Snake Antivenom Activity of Tamarindus Indica Seed Coat ExtractIJAR JOURNALNo ratings yet
- Evaluation of The Automated Coagulation Analyzer SYSMEX CA 6000Document7 pagesEvaluation of The Automated Coagulation Analyzer SYSMEX CA 6000Esther Jara GarcíaNo ratings yet
- HemophiliaDocument135 pagesHemophiliaDivyashri Baraniya100% (1)
- Anticoagulant Screening of Selected Indigenous Plant SpeciesDocument70 pagesAnticoagulant Screening of Selected Indigenous Plant SpeciesSalve Babar100% (5)
- Drug Moa PK Use Se Ci Blood Coagulation: AnticoagulantsDocument4 pagesDrug Moa PK Use Se Ci Blood Coagulation: AnticoagulantsYusoff RamdzanNo ratings yet
- Acl9000 PDFDocument391 pagesAcl9000 PDFRENE ALEJANDRO ILLESCA NEIRANo ratings yet
- XareltoDocument2 pagesXareltoMichael KuzbytNo ratings yet
- MK Hemodynamics PathologyDocument27 pagesMK Hemodynamics PathologyMoses Jr Kazevu100% (1)
- INTRODUCTION - The Two Broad Categories of Stroke, Hemorrhage and IschemiaDocument11 pagesINTRODUCTION - The Two Broad Categories of Stroke, Hemorrhage and IschemiaJose Enrique OrtizNo ratings yet
- Blood Coagulation TestDocument3 pagesBlood Coagulation TestDarell M. BookNo ratings yet
- Laboratory Evaluation of HemostasisDocument7 pagesLaboratory Evaluation of HemostasisGerly MaglangitNo ratings yet
- Body Fluids and CirculationDocument97 pagesBody Fluids and CirculationRiski BannaNo ratings yet
- Chapter 026 CoagulationDocument7 pagesChapter 026 Coagulationthubtendrolma100% (3)
- D'Dimer, Fibrinogen Dan IL6 PAD CovidDocument8 pagesD'Dimer, Fibrinogen Dan IL6 PAD CovidWartimah imahNo ratings yet
- 2934 Hemochron ManualDocument32 pages2934 Hemochron ManualFrank QuitianNo ratings yet
- CoagulationDocument3 pagesCoagulationHerho-nyl CesNo ratings yet
- MegakaryopoiesisDocument13 pagesMegakaryopoiesisAezel CruzNo ratings yet
- Science Illustrated 60 - 2018 AUDocument84 pagesScience Illustrated 60 - 2018 AUCeleborn021No ratings yet
- Chapter 33 - Assessment and Management of Patients With HematologicDocument8 pagesChapter 33 - Assessment and Management of Patients With HematologicMichael Boado100% (1)
- Coagulation CascadeDocument5 pagesCoagulation Cascadepieterinpretoria391100% (1)
- Analyticon Coagulyzer 1 2 4Document4 pagesAnalyticon Coagulyzer 1 2 4Carla YcoNo ratings yet
- Brunner and Suddarth's Textbook of Medical-Surgical NursingDocument7 pagesBrunner and Suddarth's Textbook of Medical-Surgical NursingNurseRiemNo ratings yet
- Adsorbed PlasmaDocument3 pagesAdsorbed PlasmaDevi OktaviannyNo ratings yet
- Edexcel A Level Biology Key TermsDocument2 pagesEdexcel A Level Biology Key TermsShifa RahmanNo ratings yet
- Blood Bottles GuideDocument9 pagesBlood Bottles GuideLohJNo ratings yet