Professional Documents
Culture Documents
Colloids
Colloids
Uploaded by
Acorda Angelina0 ratings0% found this document useful (0 votes)
29 views8 pagesA colloid is a mixture where particles are evenly scattered throughout a dispersed medium without settling. Flour dissolved in water forms a colloid visible due to Tyndall effect, where light scatters off dispersed particles. Colloids have many uses including natural ones like blood, and man-made ones as food ingredients, medicines, cosmetics, paints, inks, and insecticides.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
PPT, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentA colloid is a mixture where particles are evenly scattered throughout a dispersed medium without settling. Flour dissolved in water forms a colloid visible due to Tyndall effect, where light scatters off dispersed particles. Colloids have many uses including natural ones like blood, and man-made ones as food ingredients, medicines, cosmetics, paints, inks, and insecticides.
Copyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
29 views8 pagesColloids
Colloids
Uploaded by
Acorda AngelinaA colloid is a mixture where particles are evenly scattered throughout a dispersed medium without settling. Flour dissolved in water forms a colloid visible due to Tyndall effect, where light scatters off dispersed particles. Colloids have many uses including natural ones like blood, and man-made ones as food ingredients, medicines, cosmetics, paints, inks, and insecticides.
Copyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
You are on page 1of 8
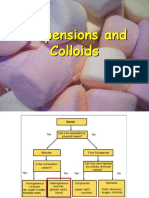
A mixture with particles evenly scattered
in a dispersed medium without settling
down is a called colloid. Investigating a
colloidal property of the mixtures: flour
dissolved in water is a colloid while
sugar dissolved in water is a solution.
The flour particles can absorb, reflect
and scatter light; therefore a beam of
light passing through the set-up was
visible.
This scattering of light is called
Tyndall Effect. Tyndall Effect is
seen as a beam of light in a
colloid because there is a
scattering of light when light
beams pass through it due to the
dispersed particles which absorb,
reflect and scatter the light.
The different examples of colloids are
important to daily life. Natural colloids
such as blood, clouds and fog are basic
for living things. Man-made colloids are
also useful. Numerous colloids such as
milk, butter, gelatin, jam, jelly, and other
creamy substances such as mayonnaise
and whipped cream, are used as food or
ingredients for preparing food.
Some colloids such as magnesium
hydroxide, creams, and ointments
are used as medicines and
cosmetics. Paints have both
protective and decorative functions.
Styrofoam, inks, and white glues are
used in offices and printing press.
Insecticides are used in farming.
A. Read the statement. Then
encircle the YES if it is correct and
NO if it is incorrect.
YES NO Colloids are homogeneous mixtures.
Light cannot pass through colloidal
YES NO particles.
The components of a colloid do not
YES NO settle at the bottom.
Colloid particles are bigger than
YES NO suspension particles.
Colloid particles are larger than
YES NO solution particles.
You might also like
- Colloidal StateDocument24 pagesColloidal Stateakash gargNo ratings yet
- Chemistry Project File For Class 12thDocument9 pagesChemistry Project File For Class 12thneovaibhav79% (111)
- SHD Form 4 Teachers Health CardDocument1 pageSHD Form 4 Teachers Health CardAcorda Angelina100% (3)
- COLLOIDSDocument33 pagesCOLLOIDSIsaiahRiveraNo ratings yet
- Sci 6 Suspensions and ColloidsDocument29 pagesSci 6 Suspensions and ColloidsDionelyn CruzNo ratings yet
- Tarpapel Lesson FormatDocument7 pagesTarpapel Lesson Formatᜃᜒᜁᜋ᜔ᜑ᜔ᜀᜈ᜔ ᜄᜓᜋᜃ᜔ᜂᜁᜎ᜔ ᜊᜃ᜔ᜁᜈᜒᜎ᜔ᜎᜓ100% (1)
- Science LessonsDocument8 pagesScience LessonsAnita PoshNo ratings yet
- Bullies, Socialites and the Shy Ones: Exploring the Personalities of Watercolor PaintsFrom EverandBullies, Socialites and the Shy Ones: Exploring the Personalities of Watercolor PaintsNo ratings yet
- Describe The Appearance and Uses of ColloidsDocument20 pagesDescribe The Appearance and Uses of ColloidsLuisa Jamero Regalado50% (2)
- SCIENCE 6 3rd QuarterDocument14 pagesSCIENCE 6 3rd QuarterMaryanne Rose ElderNo ratings yet
- Colloids: Agent To Form Stable EmulsionDocument2 pagesColloids: Agent To Form Stable EmulsionMelvin CabonegroNo ratings yet
- General Chemistry "Colloid Summary " Lectures: Elfrida Ginting, S.Si, M.SiDocument5 pagesGeneral Chemistry "Colloid Summary " Lectures: Elfrida Ginting, S.Si, M.SiGresia FalentinaNo ratings yet
- Types of MixturesDocument17 pagesTypes of MixturesMarvin De JonggoyNo ratings yet
- PPT-9C 7Document17 pagesPPT-9C 7Sneham PalangdarNo ratings yet
- Suspensions and ColloidsDocument3 pagesSuspensions and Colloidsmonster40lbsNo ratings yet
- Colloid: SolutionDocument4 pagesColloid: SolutionMark LuzNo ratings yet
- Science IM Q1 W3 D2 TYPES OF COLLOIDSDocument28 pagesScience IM Q1 W3 D2 TYPES OF COLLOIDSJeff EspiNo ratings yet
- Colloids PPSXDocument6 pagesColloids PPSXPinky Caina CabotajeNo ratings yet
- Science Grade6 Quarter1 Module3 Week3Document11 pagesScience Grade6 Quarter1 Module3 Week3Lariza LorenoNo ratings yet
- Dual Credit Research Paper RevisedDocument11 pagesDual Credit Research Paper Revisedapi-434746568No ratings yet
- Shopping For Colloids: Science-VIDocument15 pagesShopping For Colloids: Science-VIEvelynNo ratings yet
- Class 9 Chemistry Notes Chapter 2 and 4Document10 pagesClass 9 Chemistry Notes Chapter 2 and 4ap4618720No ratings yet
- 9th CH 6 EngDocument6 pages9th CH 6 EngNateqa WaqasNo ratings yet
- Solutions and Other Mixtures: Mark Denver R. Santiago, LPT 09971783372Document18 pagesSolutions and Other Mixtures: Mark Denver R. Santiago, LPT 09971783372Ramzen Raphael DomingoNo ratings yet
- Module #3 Classifications and Formation of Mixtures (New)Document9 pagesModule #3 Classifications and Formation of Mixtures (New)Bryan SorianoNo ratings yet
- Science 6 - Juzallie S. Sioco. - Colloids PDFDocument11 pagesScience 6 - Juzallie S. Sioco. - Colloids PDFJuzallie SiocoNo ratings yet
- Acids, Bases and MixturesDocument9 pagesAcids, Bases and MixtureskaylaohardyNo ratings yet
- Scie Q1 ColloidsDocument33 pagesScie Q1 Colloidscarling santiagoNo ratings yet
- INTRODUCTIONDocument11 pagesINTRODUCTIONkavya singhNo ratings yet
- ColloidsDocument31 pagesColloids12dcc1947No ratings yet
- Colloids - Introduction:: NM NMDocument6 pagesColloids - Introduction:: NM NMLipi SharmaNo ratings yet
- I Am Sharing 'SOLUTIONS (WEEK 3) ' With YouDocument6 pagesI Am Sharing 'SOLUTIONS (WEEK 3) ' With Youokohchidi1No ratings yet
- Colloids - Class 12 Chemistry Investigatory Project Free PDF DownloadDocument8 pagesColloids - Class 12 Chemistry Investigatory Project Free PDF DownloadPratyush Meher0% (1)
- Lesson 1 Homogeneous and Heterogeneous MixturesDocument28 pagesLesson 1 Homogeneous and Heterogeneous MixturesFlorenda Sorilla SampianoNo ratings yet
- Chemistry ProjectDocument27 pagesChemistry Projectbibeka Computer123No ratings yet
- Is Matter Around Us PureDocument13 pagesIs Matter Around Us PureNANDITA NAYAK BNo ratings yet
- Pure Substances and MixturesDocument15 pagesPure Substances and MixturesChrisaundria LuceroNo ratings yet
- ColloidsDocument22 pagesColloidsAditi DadhwalNo ratings yet
- ColloidsDocument19 pagesColloidsHarshNo ratings yet
- Acids and Bases HandoutDocument2 pagesAcids and Bases HandoutSohid BacusNo ratings yet
- ColloidDocument10 pagesColloidRohit ChoudharyNo ratings yet
- Chem M5 ColloidsDocument19 pagesChem M5 ColloidsEstrie Niku Sanes JaleerNo ratings yet
- Surface Chemistry-2Document33 pagesSurface Chemistry-2Firdha Aulia Noor FadilahNo ratings yet
- Chapter 2 - Matter Around Us PureDocument14 pagesChapter 2 - Matter Around Us PureSwathi KarunakaranNo ratings yet
- Colloid & Emulsion ExperimentDocument2 pagesColloid & Emulsion ExperimentFaizal Ashrafi Bugo100% (1)
- WWW Askiitians Com Revision Notes Class 9 Science Is Matter Around Us PureDocument17 pagesWWW Askiitians Com Revision Notes Class 9 Science Is Matter Around Us PureDeepak Kumar SharmaNo ratings yet
- Tyndall EffectDocument3 pagesTyndall EffectMelissa A. BernardoNo ratings yet
- Chapter 17 Lesson 1Document20 pagesChapter 17 Lesson 1bm9gvfxj6hNo ratings yet
- Colloid and Surface Science: Nurul Huda BT Abu Bakar 012-7892664 Hudabakar@ump - Edu.myDocument8 pagesColloid and Surface Science: Nurul Huda BT Abu Bakar 012-7892664 Hudabakar@ump - Edu.myAlbert12346No ratings yet
- Particle Che572 PDFDocument5 pagesParticle Che572 PDFaimanshibliNo ratings yet
- Colloids: Describe The Characteristics and Uses of Simple ColloidsDocument19 pagesColloids: Describe The Characteristics and Uses of Simple Colloidscatherine renanteNo ratings yet
- Colloids: Describe The Characteristics and Uses of Simple ColloidsDocument18 pagesColloids: Describe The Characteristics and Uses of Simple Colloidscatherine renanteNo ratings yet
- Science Lessons 3 To 6 (Autosaved)Document24 pagesScience Lessons 3 To 6 (Autosaved)Rodgen GerasolNo ratings yet
- Is Matter Around Us PureDocument21 pagesIs Matter Around Us PureKunalNo ratings yet
- Chemistry - Solutions and Their BehaviorDocument48 pagesChemistry - Solutions and Their BehaviorMohdErwanNo ratings yet
- G6Q1W5 6Document38 pagesG6Q1W5 6Shiella Mariz BinotapaNo ratings yet
- Suspensions and Colloids NotesDocument3 pagesSuspensions and Colloids Noteskallstea100% (1)
- ColloidsDocument5 pagesColloidsShahid Ullah100% (1)
- Coconut Oil IsDocument2 pagesCoconut Oil IsYen BumNo ratings yet
- Coll OidsDocument61 pagesColl OidsSk KalamNo ratings yet
- Oil and Water Won't Mix and Other Mixture Separation Techniques - Chemistry Book for Kids 8-10 | Children's Chemistry BooksFrom EverandOil and Water Won't Mix and Other Mixture Separation Techniques - Chemistry Book for Kids 8-10 | Children's Chemistry BooksNo ratings yet
- Addendum To Division Memo On The Ranking of Applicants For Head Teachers I-IIIDocument1 pageAddendum To Division Memo On The Ranking of Applicants For Head Teachers I-IIIAcorda AngelinaNo ratings yet
- Division Memo No 17-40, S. 2016Document1 pageDivision Memo No 17-40, S. 2016Acorda AngelinaNo ratings yet
- BRS PreparationDocument16 pagesBRS PreparationAcorda AngelinaNo ratings yet
- Math New DLL November 28-December 2 2016, 2016 Marianne Puhi-2ndDocument14 pagesMath New DLL November 28-December 2 2016, 2016 Marianne Puhi-2ndAcorda AngelinaNo ratings yet
- ESP Nov. 15Document2 pagesESP Nov. 15Acorda AngelinaNo ratings yet
- Grade 3 1st GradingDocument3 pagesGrade 3 1st GradingAcorda AngelinaNo ratings yet
- Division Memorandum No. 351, s.2020Document2 pagesDivision Memorandum No. 351, s.2020Acorda AngelinaNo ratings yet
- Department of Education: Republic of The PhilippinesDocument1 pageDepartment of Education: Republic of The PhilippinesAcorda AngelinaNo ratings yet
- 1 - English - FirstGrading-Recognize - Identify and Classify Transfortation Sound As Loud and SoftDocument16 pages1 - English - FirstGrading-Recognize - Identify and Classify Transfortation Sound As Loud and SoftAcorda AngelinaNo ratings yet
- Swimming: This Photo CC By-Nc-NdDocument17 pagesSwimming: This Photo CC By-Nc-NdAcorda AngelinaNo ratings yet
- Estimate Each SumDocument21 pagesEstimate Each SumAcorda AngelinaNo ratings yet
- Day 15 - DecantationDocument13 pagesDay 15 - DecantationAcorda AngelinaNo ratings yet
- Day 13 - Medicinal PlantsDocument7 pagesDay 13 - Medicinal PlantsAcorda AngelinaNo ratings yet
- Day 17 - SievingDocument12 pagesDay 17 - SievingAcorda AngelinaNo ratings yet
- Day 5 - MixtureDocument11 pagesDay 5 - MixtureAcorda AngelinaNo ratings yet
- Day 4 - Acid Vs BaseDocument14 pagesDay 4 - Acid Vs BaseAcorda AngelinaNo ratings yet
- Day 12 - Useful DrinksDocument7 pagesDay 12 - Useful DrinksAcorda AngelinaNo ratings yet