Professional Documents
Culture Documents
Respiration 3: Dr. Bushra Sultana Assistant Professor Iirs
Uploaded by
Natasha0 ratings0% found this document useful (0 votes)
3 views23 pagesOriginal Title
Respiration 3
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
3 views23 pagesRespiration 3: Dr. Bushra Sultana Assistant Professor Iirs
Uploaded by
NatashaCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You are on page 1of 23
RESPIRATION 3
Dr. Bushra Sultana
Assistant Professor
IIRS
Physical Principles of Gas
Exchange; Diffusion of Oxygen
and Carbon Dioxide Through
the Respiratory Membrane
• After the alveoli are ventilated with fresh air, the next step in the
respiratory process is diffusion of oxygen from the alveoli into the
pulmonary blood and diffusion of carbon dioxide in the opposite
direction, out of the blood. The process of diffusion is simply the
random motion of molecules intertwining their way in all directions
through the respiratory membrane and adjacent fluids.
Physics of Gas Diffusion and Gas
Partial Pressures
• All the gases of concern in respiratory physiology are simple molecules that
are free to move among one another, which is the process called
“diffusion.”This is also true of gases dissolved in the fluids and tissues of the
body.
• For diffusion to occur, there must be a source of energy. This is provided by
the kinetic motion of the molecules themselves. Except at absolute zero
temperature, all molecules of all matter are continually undergoing motion.
For free molecules that are not physically attached to others, this means
linear movement at high velocity until they strike other molecules. Then they
bounce away in new directions and continue until striking other molecules
again. In this way, the molecules move rapidly and randomly among one
another.
Net Diffusion of a Gas in One Direction—
Effect of a Concentration Gradient.
• If a gas chamber or a solution has a high concentration of a particular
gas at one end of the chamber and a low concentration at the other
end, net diffusion of the gas will occur from the high-concentration
area toward the low-concentration area.The reason is obvious:There
are far more molecules at end A of the chamber to diffuse toward end
B than there are molecules to diffuse in the opposite direction.
• Therefore, the rates of diffusion in each of the two directions are
proportionately different, as demonstrated by the lengths of the
arrows in the figure.
Gas Pressures in a Mixture of Gases
—“Partial Pressures” of Individual Gases
• Pressure is caused by multiple impacts of moving molecules against a
surface.Therefore, the pressure of a gas acting on the surfaces of the
respiratory passages and alveoli is proportional to the summated
force of impact of all the molecules of that gas striking the surface at
any given instant. This means that the pressure is directly proportional
to the concentration of the gas molecules.
• In respiratory physiology, one deals with mixtures of gases, mainly of
oxygen, nitrogen, and carbon dioxide. The rate of diffusion of each of
these gases is directly proportional to the pressure caused by that gas
alone, which is called the partial pressure of that gas.
Pressures of Gases Dissolved in
Water and Tissues
• Gases dissolved in water or in body tissues also exert pressure,
because the dissolved gas molecules are moving randomly and have
kinetic energy. Further, when the gas dissolved in fluid encounters a
surface, such as the membrane of a cell, it exerts its own partial
pressure in the same way that a gas in the gas phase does.
• The partial pressures of the separate dissolved gases are designated
the same as the partial pressures in the gas state, that is, Po2, Pco2,
Pn2, and so forth.
Factors That Determine the Partial
Pressure of a Gas Dissolved in a Fluid.
• The partial pressure of a gas in a solution is determined not only by its
concentration but also by the solubility coefficient of the gas. That is,
some types of molecules, especially carbon dioxide, are physically or
chemically attracted to water molecules, whereas others are repelled.
When molecules are attracted, far more of them can be dissolved
without building up excess partial pressure within the solution.
Conversely, in the case of those that are repelled, high partial
pressure will develop with fewer dissolved molecules. These relations
are expressed by the following formula, which is Henry’s law:
Vapor Pressure of Water
• The partial pressure that the water molecules exert to escape through the
surface is called the vapor pressure of the water.
• At normal body temperature, 37°C, this vapor pressure is 47 mm Hg.
Therefore, once the gas mixture has become fully humidified—that is,
once it is in “equilibrium” with the water—the partial pressure of the
water vapor in the gas mixture is 47 mm Hg.This partial pressure, like the
other partial pressures, is designated Ph2o.
• The vapor pressure of water depends entirely on the temperature of the
water. The greater the temperature, the greater the kinetic activity of the
molecules and, therefore, the greater the likelihood that the water
molecules will escape from the surface of the water into the gas phase.
Diffusion of Gases Through
Fluids—Pressure Difference Cause Net Diffusion
• when the partial pressure of a gas is greater in one area than in
another area, there will be net diffusion from the high-pressure area
toward the low-pressure area.
• The net diffusion of gas from the area of high pressure to the area of
low pressure is equal to the number of molecules bouncing in this
forward direction minus the number bouncing in the opposite
direction; this is proportional to the gas partial pressure difference
between the two areas, called simply the pressure difference for
causing diffusion
Quantifying the Net Rate of Diffusion in
Fluids.
• In addition to the pressure difference, several other factors affect the
rate of gas diffusion in a fluid. They are
(1) the solubility of the gas in the fluid,
(2) the cross-sectional area of the fluid,
(3) the distance through which the gas must diffuse,
(4) the molecular weight of the gas,
(5) the temperature of the fluid. In the body, the last of these factors,
the temperature, remains reasonably constant and usually need not
be considered
Composition of Alveolar Air—Its Relation to
Atmospheric Air
• Alveolar air is different from atmospheric air in the following points:
1. Alveolar air is only partially replaced by atmospheric air with each
breath.
2. Oxygen is constantly being absorbed by the alveolar air.
3. CO2 is constantly diffusing from the pulmonary blood into the
alveoli.
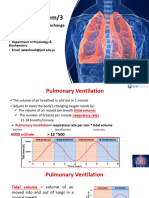
Rate at Which Alveolar Air Is Renewed
by Atmospheric Air
• the volume of alveolar air replaced by new atmospheric air with each
breath is only one seventh of the total, so that multiple breaths are
required to exchange most of the alveolar air. Figure 39–2 shows this
slow rate of renewal of the alveolar air. In the first alveolus of the
figure, an excess amount of a gas is present in the alveoli, but note
that even at the end of 16 breaths, the excess gas still has not been
completely removed from the alveoli.
Importance of the Slow Replacement of
Alveolar Air.
• The slow replacement of alveolar air is of particular importance in
preventing sudden changes in gas concentrations in the blood.
Oxygen Concentration and Partial
Pressure in the Alveoli
• Oxygen is continually being absorbed from the alveoli into the blood of the
lungs, and new oxygen is continually being breathed into the alveoli from
the atmosphere.
• The more rapidly oxygen is absorbed, the lower its concentration in the
alveoli becomes; conversely, the more rapidly new oxygen is breathed into
the alveoli from the atmosphere, the higher its concentration becomes.
• Therefore, oxygen concentration in the alveoli, and its partial pressure as
well, is controlled by
(1) the rate of absorption of oxygen into the blood and
(2) the rate of entry of new oxygen into the lungs by the ventilatory process.
CO2 Concentration and Partial
Pressure in the Alveoli
• Carbon dioxide is continually being formed in the body and then
carried in the blood to the alveoli; it is continually being removed
from the alveoli by ventilation.
• Normal rate of alveolar ventilation for CO2 is 4.2ml/min, While CO2
pressure in alveoli is 40mmHg.
• The alveolar PCO2 increases directly proportional to the rate of
carbon dioxide excretion.
• Alveolar PCO2 decreases in inverse proportion to the alveolar
ventilation.
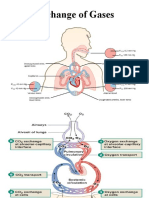
Diffusion of Gases Through
the Respiratory Membrane
• respiratory unit (also called “respiratory lobule”), which is composed
• of a respiratory bronchiole, alveolar ducts, atria, and alveoli. There are
about 300 million alveoli in the two lungs, and each alveolus has an average
diameter of about 0.2 millimeter. The alveolar walls are extremely thin, and
between the alveoli is an almost solid network of interconnecting capillaries,
Indeed, because of the extensiveness of the capillary plexus, the flow of
blood in the alveolar wall has been described as a “sheet” of flowing blood
• gas exchange between the alveolar air and the pulmonary blood occurs
through the membranes of all the terminal portions of the lungs, not merely
in the alveoli themselves. All these membranes are collectively known as the
respiratory membrane, also called the pulmonary membrane.
Respiratory Membrane
• following different layers of the respiratory membrane:
1. A layer of fluid lining the alveolus and containing surfactant that reduces
the surface tension of the alveolar fluid
2. The alveolar epithelium composed of thin epithelial cells
3. An epithelial basement membrane
4. A thin interstitial space between the alveolar epithelium and the capillary
membrane
5.A capillary basement membrane that in many places fuses with the alveolar
epithelial basement membrane
6. The capillary endothelial membrane
Ventilation Perfusion Ratio
• The ration of alveolar ventilation to the blood flow.
VPR= VA/Q
VA = Alveolar ventilation
Q= Blood flow
When VPR is zero
• It means VPR is without any alveolar ventilation because the air in the
alveolus has to come to equilibirium along with the blood oxygen and
CO2 gas.
When VPR equals infinity
• It means there is no capillary blood flow that carry oxygen away and
CO2 to the alveoli.
• Air is inspired loses no oxygen to the blood and gain no CO2.
You might also like
- Answer Ndeb Released Questions 2013 AfkDocument1 pageAnswer Ndeb Released Questions 2013 AfkKareem Shawa33% (6)
- Functions of The Respiratory SysteDocument5 pagesFunctions of The Respiratory SysteMohd FirdausNo ratings yet
- Summer Vacation Homework for Grade 6Document8 pagesSummer Vacation Homework for Grade 6zoha khanNo ratings yet
- Gaseous Exchange.. REspirationDocument34 pagesGaseous Exchange.. REspirationAmir NangyalNo ratings yet
- Partial PressureDocument75 pagesPartial PressureDrAbhilasha SharmaNo ratings yet
- Diffusion of Gases: Brig (R) Ali Nasre AlamDocument22 pagesDiffusion of Gases: Brig (R) Ali Nasre AlamSalman KhanNo ratings yet
- Difusi O2co2gas ExchangeDocument50 pagesDifusi O2co2gas ExchangeDewi SofyanaNo ratings yet
- Principles of Gas Exchange Diffusionof Oxygen and Carbon Dioxide Through The Respiratory MembraneDocument23 pagesPrinciples of Gas Exchange Diffusionof Oxygen and Carbon Dioxide Through The Respiratory MembraneSAKARIYE MAXAMEDNo ratings yet
- Respiratory System/3: Physical Principles of Gas ExchangeDocument27 pagesRespiratory System/3: Physical Principles of Gas ExchangeMohammad AlomariNo ratings yet
- Gas Exchange: Understanding Diffusion & Partial PressuresDocument15 pagesGas Exchange: Understanding Diffusion & Partial Pressuressoleha723No ratings yet
- Vpy I KP Paudel Laws Related To Solubility of Gas, Physical Principles of Gas Exchange and Exchange of Gases in Lungs and TissuesDocument38 pagesVpy I KP Paudel Laws Related To Solubility of Gas, Physical Principles of Gas Exchange and Exchange of Gases in Lungs and TissuesKNo ratings yet
- Module 19 Respiratory FinalDocument12 pagesModule 19 Respiratory FinalJayR MendonesNo ratings yet
- Gas ExchangeDocument5 pagesGas Exchangekeerthi krishnaNo ratings yet
- RESP CH 3 Principles of Gas ExchangeDocument66 pagesRESP CH 3 Principles of Gas ExchangeAhmed khanNo ratings yet
- Exam 4 NotesDocument54 pagesExam 4 NotesAnonymous qaAbg65dCNo ratings yet
- Assignment Gaseous ExchangeDocument10 pagesAssignment Gaseous ExchangefetramyraNo ratings yet
- VENTILATIONDocument27 pagesVENTILATIONezeudunicholas16No ratings yet
- Lecture 6 Respiratory System 2Document33 pagesLecture 6 Respiratory System 2hafiz patahNo ratings yet
- Bio Notes (B3.1) Tuesday 30TH JanuaryDocument11 pagesBio Notes (B3.1) Tuesday 30TH JanuaryNeema Sarah MUTWIRINo ratings yet
- Pulmonary VentilationDocument12 pagesPulmonary Ventilationtan shin meiNo ratings yet
- Lecture 2-8 - Gases Exchange Regulation of RespirationDocument32 pagesLecture 2-8 - Gases Exchange Regulation of RespirationCynxNo ratings yet
- Physiology of Respiration 2Document31 pagesPhysiology of Respiration 2kuhutansittinurhalizaNo ratings yet
- Respiratory Physiology Part 1, Revised 2020Document48 pagesRespiratory Physiology Part 1, Revised 2020Allan Q Venus100% (1)
- Respiratory System Gas ExchangeDocument54 pagesRespiratory System Gas ExchangeOdunfa OlufeyikemiNo ratings yet
- Gas exchange in the lungs: surface area and diffusionDocument30 pagesGas exchange in the lungs: surface area and diffusionYasasviNo ratings yet
- Mechanism of RespirationDocument18 pagesMechanism of RespirationHoney BoneyNo ratings yet
- Reflection Respiratory SystemDocument4 pagesReflection Respiratory SystemAin Sufiza0% (1)
- Kuliah Icsada Gas ExchangeDocument76 pagesKuliah Icsada Gas ExchangeAndy Pranata KusumaNo ratings yet
- Respiratory System Uploaded On Dow PortalDocument52 pagesRespiratory System Uploaded On Dow PortalAyesha MasoodNo ratings yet
- Function of The Respiratory SystemDocument5 pagesFunction of The Respiratory SystemRachelHatcher123No ratings yet
- RespirationsDocument31 pagesRespirationsEdward Munyaradzi KutsanziraNo ratings yet
- University of Guyana School of Medicine Med 1106 - Physiology I DR Kalima ThompsonDocument66 pagesUniversity of Guyana School of Medicine Med 1106 - Physiology I DR Kalima ThompsonKNo ratings yet
- Respiratory System: A Tutorial Study GuideFrom EverandRespiratory System: A Tutorial Study GuideRating: 5 out of 5 stars5/5 (1)
- Respiratory PhysiologyDocument37 pagesRespiratory PhysiologyclarisseNo ratings yet
- Bio 215 Topic 7 RespiratoryDocument46 pagesBio 215 Topic 7 RespiratoryYara BeainiNo ratings yet
- Compliance of LungsDocument37 pagesCompliance of LungsJasir KhanNo ratings yet
- Chapter 12Document28 pagesChapter 12براءة أحمد السلاماتNo ratings yet
- Chapter 12Document28 pagesChapter 12Ammar SmadiNo ratings yet
- Human Respiratory System ExplainedDocument4 pagesHuman Respiratory System ExplainedSheehan MathurNo ratings yet
- Gas Exchange in Animals PDFDocument4 pagesGas Exchange in Animals PDFmunzarin100% (1)
- Oxygen Delivery Process in 6 Simple StepsDocument10 pagesOxygen Delivery Process in 6 Simple StepsFitri FebriantiNo ratings yet
- 24 - Physiology of The Respiratory System PDFDocument38 pages24 - Physiology of The Respiratory System PDFlovelyc95No ratings yet
- Exchange of GasesDocument14 pagesExchange of GasesSudhaNo ratings yet
- Gas Exchange & Partial Pressure RegulationDocument7 pagesGas Exchange & Partial Pressure Regulationrienz nicnic peraltaNo ratings yet
- The Respiratory SystemDocument2 pagesThe Respiratory SystemconnievelardeNo ratings yet
- Dr. Sunita Saxena - Breathing and Exchange of GassesDocument48 pagesDr. Sunita Saxena - Breathing and Exchange of GassesDivya AgarawalNo ratings yet
- Q1-M1-KMT and Gas Laws (BL)Document34 pagesQ1-M1-KMT and Gas Laws (BL)Jim AñonuevoNo ratings yet
- Biofluid Mechanics Chapter 3Document35 pagesBiofluid Mechanics Chapter 3AbcdNo ratings yet
- RespirationDocument32 pagesRespirationAndersonNo ratings yet
- Chapter 5: GasesDocument78 pagesChapter 5: GasesDucklingduckNo ratings yet
- The Respiratory System ExplainedDocument28 pagesThe Respiratory System ExplainedBana ItanNo ratings yet
- Gaseous ExchangeDocument23 pagesGaseous ExchangenikitaNo ratings yet
- Altitude Physiology: PRESENTED BY Dick WilliamsDocument52 pagesAltitude Physiology: PRESENTED BY Dick WilliamsDeepak ArkalgudNo ratings yet
- The Respiratory SystemDocument2 pagesThe Respiratory SystemRaluca MoldovanNo ratings yet
- 3 Gaseous Exchange Through The Respiratory Membrane.Document23 pages3 Gaseous Exchange Through The Respiratory Membrane.Ahmed AliNo ratings yet
- Respi PhysiologyDocument55 pagesRespi Physiologymutthineni.sushma28No ratings yet
- Respiratory PhysiologyDocument39 pagesRespiratory Physiologytesfamichael mengistuNo ratings yet
- Chapter 6. Diffusion of Gases and Interpretation of Pulmonary Function TestsDocument11 pagesChapter 6. Diffusion of Gases and Interpretation of Pulmonary Function Testsdrnasir31No ratings yet
- Gas Laws in RespirationDocument3 pagesGas Laws in Respirationroberto543No ratings yet
- Alveolar Gas ExchangeDocument16 pagesAlveolar Gas ExchangeAndy AgyemangNo ratings yet
- Respiratory Monitoring in Mechanical Ventilation: Techniques and ApplicationsFrom EverandRespiratory Monitoring in Mechanical Ventilation: Techniques and ApplicationsJian-Xin ZhouNo ratings yet
- High Impedance Fault Detection On Rural Electric Distribution and Power Quality Control SystemsDocument4 pagesHigh Impedance Fault Detection On Rural Electric Distribution and Power Quality Control SystemsGerman MonjeNo ratings yet
- Video Decoder For Portable LCD Display: VP77 Data SheetDocument72 pagesVideo Decoder For Portable LCD Display: VP77 Data Sheetcarlos cardenasNo ratings yet
- r050212101 Object Oriented Analysis Design Through UmlDocument8 pagesr050212101 Object Oriented Analysis Design Through UmlSrinivasa Rao GNo ratings yet
- Why Single-Ended Tube AmplifiersDocument17 pagesWhy Single-Ended Tube Amplifiersjimmy67music100% (2)
- 12TH CS TERM 2 - PythonDocument8 pages12TH CS TERM 2 - PythonAnbuchelvan VKNo ratings yet
- ChemistryDocument84 pagesChemistryMaria Regina SantosNo ratings yet
- Periodic table elements in Chinese charactersDocument3 pagesPeriodic table elements in Chinese charactersTheodore HaralabisNo ratings yet
- 03 - Reactions Between CaO and SO2 in Carbonating and No Carbonating ConditionsDocument9 pages03 - Reactions Between CaO and SO2 in Carbonating and No Carbonating ConditionsNishantNo ratings yet
- Insulated Pipe ClampsDocument17 pagesInsulated Pipe ClampsKarun NayyarNo ratings yet
- Lightweight Universal Formwork For Walls, Slabs, Columns and FoundationsDocument52 pagesLightweight Universal Formwork For Walls, Slabs, Columns and FoundationsKrishiv ChanglaniNo ratings yet
- National Talent Search Examination Path to SuccessDocument25 pagesNational Talent Search Examination Path to SuccessSudhanshuNo ratings yet
- Mac Green Button EN PDFDocument5 pagesMac Green Button EN PDFVanessa ManriqueNo ratings yet
- Manual de Utilizare Sursa de Alimentare 27.6 V5 A Pulsar EN54-5A17 230 VAC50 HZ Montaj Aparent LEDDocument40 pagesManual de Utilizare Sursa de Alimentare 27.6 V5 A Pulsar EN54-5A17 230 VAC50 HZ Montaj Aparent LEDGabriel SerbanNo ratings yet
- Manual Aid 2Document136 pagesManual Aid 2Luis Chinchilla CruzNo ratings yet
- Pureroutines: SmoothnessDocument11 pagesPureroutines: SmoothnessJohn PauloNo ratings yet
- Introduction To Photogrammetry With UAVDocument107 pagesIntroduction To Photogrammetry With UAVut4ulu100% (1)
- 1992 - Niobium Additions in HP Heat-Resistant Cast Stainless SteelsDocument10 pages1992 - Niobium Additions in HP Heat-Resistant Cast Stainless SteelsLuiz Gustavo LimaNo ratings yet
- Zeek Case StudyDocument4 pagesZeek Case StudysuryanshNo ratings yet
- Map Grid and Compass RoseDocument3 pagesMap Grid and Compass RosePatricia BelangerNo ratings yet
- Application Notes UC3710TDocument16 pagesApplication Notes UC3710TSHAHID_71No ratings yet
- Application of DerivativesDocument53 pagesApplication of DerivativesTushar PalNo ratings yet
- Geo technical engineering fundamentalsDocument44 pagesGeo technical engineering fundamentalsSri Gaja Govind Babu100% (1)
- Math Cheat SheetDocument33 pagesMath Cheat SheetSanjeevG100% (6)
- Pipe Network Analysis: Chemical Engineer's GuideDocument12 pagesPipe Network Analysis: Chemical Engineer's GuideRio BuiNo ratings yet
- Introduction To NanophotonicsDocument49 pagesIntroduction To NanophotonicsArashiNo ratings yet
- Data For Tightening Torque: LubricantDocument5 pagesData For Tightening Torque: LubricantAtanasio PerezNo ratings yet
- Geeks For Geeks - ArrayDocument33 pagesGeeks For Geeks - ArrayLove MehtaNo ratings yet
- 1993 Engines Eurovan - 2.5L 5-CylinderDocument20 pages1993 Engines Eurovan - 2.5L 5-Cylinderfrancesco pavanNo ratings yet