Professional Documents
Culture Documents
Kidneys' Role in Maintaining Acid-Base Balance
Uploaded by
Prakash Panthi0 ratings0% found this document useful (0 votes)
35 views18 pagesThe kidneys play a key role in acid-base balance by reabsorbing bicarbonate ions and secreting hydrogen ions to counteract acidosis, and secreting bicarbonate ions while reabsorbing hydrogen ions to counteract alkalosis. The kidneys maintain pH through two main functions - reabsorbing all filtered bicarbonate principally in the proximal tubule, and excreting the daily hydrogen ion load in the collecting duct. Through mechanisms like generating new bicarbonate ions while excreting buffered hydrogen ions, and excreting ammonium ions which produce bicarbonate, the kidneys are ultimately responsible for regulating acid-base balance.
Original Description:
Kidneys role in Acid-Base balance
Original Title
Role_of_kidney_in_acid_base_balance(4)
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe kidneys play a key role in acid-base balance by reabsorbing bicarbonate ions and secreting hydrogen ions to counteract acidosis, and secreting bicarbonate ions while reabsorbing hydrogen ions to counteract alkalosis. The kidneys maintain pH through two main functions - reabsorbing all filtered bicarbonate principally in the proximal tubule, and excreting the daily hydrogen ion load in the collecting duct. Through mechanisms like generating new bicarbonate ions while excreting buffered hydrogen ions, and excreting ammonium ions which produce bicarbonate, the kidneys are ultimately responsible for regulating acid-base balance.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
35 views18 pagesKidneys' Role in Maintaining Acid-Base Balance
Uploaded by
Prakash PanthiThe kidneys play a key role in acid-base balance by reabsorbing bicarbonate ions and secreting hydrogen ions to counteract acidosis, and secreting bicarbonate ions while reabsorbing hydrogen ions to counteract alkalosis. The kidneys maintain pH through two main functions - reabsorbing all filtered bicarbonate principally in the proximal tubule, and excreting the daily hydrogen ion load in the collecting duct. Through mechanisms like generating new bicarbonate ions while excreting buffered hydrogen ions, and excreting ammonium ions which produce bicarbonate, the kidneys are ultimately responsible for regulating acid-base balance.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You are on page 1of 18
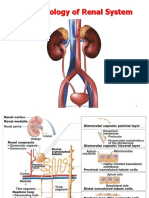
Role of kidney in Acid-Base Balance
Only the kidneys can rid the body of acids generated
by cellular metabolism (nonvolatile or fixed acids)
• The kidney in response:
–To Acidosis
• Retains bicarbonate ions and eliminates hydrogen
ions
–To Alkalosis
• Eliminates bicarbonate ions and retains
hydrogen ions
To maintain normal pH, the kidneys must
perform 2 physiologic functions.
1.Reabsorb all the filtered HCO3:
• A function principally of the proximal tubule.
2. To excrete the daily H+ load:
• A function of the collecting duct.
Chemical buffers can tie up excess acids or bases, but
they cannot eliminate them from the body.
The lungs can eliminate carbonic acid by eliminating
carbon dioxide.
Only the kidneys can rid the body of metabolic acids
(phosphoric, uric, and lactic acids and ketones) and
prevent metabolic acidosis.
The ultimate acid-base regulatory organs are the
kidneys.
The most important renal mechanisms for
regulating acid-base balance are conserving
(reabsorbing) or generating new bicarbonate ions
and excreting bicarbonate ions
Losing a bicarbonate ion is the same as gaining a
hydrogen ion; reabsorbing a bicarbonate ion is
the same as losing a hydrogen ion.
Reabsorption of Bicarbonate:
Plasma bicarbonate is freely filtered at the
glomerulus.
Carbonic acid formed in filtrate dissociates to
release carbon dioxide and water
Carbon dioxide then diffuses into tubule cells,
where it acts to trigger further hydrogen ion
secretion.
For each hydrogen ion secreted, a sodium ion
and a bicarbonate ion are reabsorbed by the
PCT cells
Secreted hydrogen ions form carbonic acid.
Thus, bicarbonate disappears from filtrate at the
same rate that it enters the peritubular capillary
blood.
Reabsorption of Bicarbonate Ions:
Generating New Bicarbonate Ions:
Two mechanisms carried out by tubule cells
generate new bicarbonate ions
Both involve renal excretion of acid via secretion
and excretion of hydrogen ions or ammonium
ions (NH4+)
Excretion Of Buffered H Ions:
Alpha intercalated cells of the renal tubules
can synthesize new bicarbonate ions while
excreting more hydrogen ions.
Excretion Of Buffered H+:
In response to acidosis hydrogen ions must be
counteracted by generating new bicarbonate
Kidneys generate bicarbonate ions and add them to the
blood
An equal amount of hydrogen ions are added to the
urine
Dietary:The excreted hydrogen ions must bind to
buffers (phosphate buffer system) in the urine and
excreted.
Bicarbonate generated is then moved into the
interstitial space via a cotransport system
Passively moved into the peritubular capillary
blood
Synthesis Of New Bicarbonate & Excretion Of Buffered H+:
Excretion Of Ammonium Ion :
Ammonium ions are weak acids.
This method uses ammonium ions produced by the
metabolism of glutamine in PCT cells.
Each glutamine metabolized produces two ammonium
ions and two bicarbonate ions.
Bicarbonate moves to the blood and ammonium ions
are excreted in urine.
NH4+ Excretion :
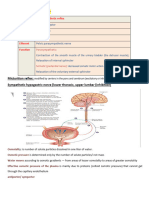
Bicarbonate Ion Secretion
When the body is in alkalosis, tubular cells
secrete bicarbonate ions and reclaim hydrogen
ions and acidify the blood.
This mechanism is the opposite of bicarbonate
ion reabsorption process.
Bicarbonate Ion Secretion
Daily Reabsorption of HCO-3 By Kidney:
85% HCO3 reabsorption (H+ Secretion) occurs in
PCT (proximal convoluted tubule)
10% HCO3 reabsorption (H+ secretion)occurs in thick
ascending LOH
4.9% (approx 5%) reabsorption (H+ secretion) occurs
in DCT & CT.
For each HCO3 reabsorbed, there must be one H+ ion
secreted.
You might also like
- Quiz BiochemistryDocument100 pagesQuiz BiochemistryMedShare88% (25)
- Subconjunctival HaemorrhageDocument7 pagesSubconjunctival HaemorrhageTry Ahmad MirzaNo ratings yet
- Physiology SmellDocument22 pagesPhysiology SmellPrakash PanthiNo ratings yet
- Pharmacology of Renal SystemDocument125 pagesPharmacology of Renal SystemBirhanu GetaNo ratings yet
- Acid-Base Balance ExplainedDocument8 pagesAcid-Base Balance ExplainedNicole TangcoNo ratings yet
- The Wave Genetics as a New Understanding of How Our Genetic Apparatus WorksDocument18 pagesThe Wave Genetics as a New Understanding of How Our Genetic Apparatus WorksNorman Imperial100% (2)
- Acid Base Balance OR OR Regulation of Blood PHDocument175 pagesAcid Base Balance OR OR Regulation of Blood PHhirendra patel100% (1)
- Daily Acid Load RegulationDocument11 pagesDaily Acid Load RegulationPrastia StratosNo ratings yet
- Renal Control of Acid Base BalanceDocument24 pagesRenal Control of Acid Base BalanceFarryrazaNo ratings yet
- Renal Control of Acid Base BalanceDocument5 pagesRenal Control of Acid Base BalanceEuniceSimNo ratings yet
- The Kidneys Role in Maintaining Acid-Base HomeostasisDocument17 pagesThe Kidneys Role in Maintaining Acid-Base HomeostasisMaggieLocke100% (1)
- Renal-Acid Base BalanceDocument31 pagesRenal-Acid Base BalanceMai Kutin KoakNo ratings yet
- Acid Base TutorialDocument47 pagesAcid Base TutorialDiah Puspita Rini100% (2)
- Acid Base PhysiologyDocument54 pagesAcid Base PhysiologyDiana AyónNo ratings yet
- Regulation of Acid-Base BalanceDocument4 pagesRegulation of Acid-Base BalanceMich Therese Abejero100% (1)
- ACID-BASE REGULATION OVERVIEWDocument24 pagesACID-BASE REGULATION OVERVIEWshintaaaaaNo ratings yet
- Role of Kidneys in The Regulation of Acid-Base BalanceDocument83 pagesRole of Kidneys in The Regulation of Acid-Base BalanceBea SamonteNo ratings yet
- Acid-Base Balance: Normal PH of Body FluidsDocument30 pagesAcid-Base Balance: Normal PH of Body FluidsAnnDianaJaiminNo ratings yet
- Acid Base BalanceDocument35 pagesAcid Base BalanceDhanasvi Dessai100% (1)
- 11 Pengaturan Asam BasaDocument24 pages11 Pengaturan Asam BasaFandoko ChaniagoNo ratings yet
- ACID BASE BALANCE ModifiedDocument56 pagesACID BASE BALANCE ModifiedBiserat GetnetNo ratings yet
- Acid Base Regulation and Its DisordersDocument80 pagesAcid Base Regulation and Its DisordersPirate CoolNo ratings yet
- Acid - Base BalancesDocument68 pagesAcid - Base Balancesjames makulaNo ratings yet
- 4acid Base Balance and Renal Function TestsDocument50 pages4acid Base Balance and Renal Function TestsOsama MohamedNo ratings yet
- Acidification of UrineDocument14 pagesAcidification of UrineMohan BNo ratings yet
- Acid-Base Balance: Editor: Dr. Husnil Kadri, MkesDocument66 pagesAcid-Base Balance: Editor: Dr. Husnil Kadri, MkesDwi SiregarNo ratings yet
- How Bicarbonate Maintains Acid-Base BalanceDocument3 pagesHow Bicarbonate Maintains Acid-Base BalanceMalikkahNo ratings yet
- 835 Regulation of Acid Base Balance 2019Document26 pages835 Regulation of Acid Base Balance 2019sanofazal786No ratings yet
- Acidification of UrineDocument2 pagesAcidification of UrineDani ThomasNo ratings yet
- 9975 - Tutorial 4 Blok 1.3 (Akibat Diare)Document37 pages9975 - Tutorial 4 Blok 1.3 (Akibat Diare)sri rahma lizaNo ratings yet
- Acid Base BalanceDocument5 pagesAcid Base BalanceAnila zafarNo ratings yet
- The Kidneys and Formation of Urine 3 New 0Document22 pagesThe Kidneys and Formation of Urine 3 New 0Iph anyiNo ratings yet
- #2 Acid-Basic Balance in Animal BodyDocument22 pages#2 Acid-Basic Balance in Animal BodyYodi MainantoNo ratings yet
- Acid Base Regulation - ppt@BVSCAH-6th SemDocument95 pagesAcid Base Regulation - ppt@BVSCAH-6th SemDr. Prakash PanthiNo ratings yet
- Water: PH and BuffersDocument63 pagesWater: PH and BuffersSoffa ShmuelNo ratings yet
- Blood Gases, PH, and Buffer SystemDocument22 pagesBlood Gases, PH, and Buffer SystemtabletvodaNo ratings yet
- Haemoglobin Acts As Acid Base SystemDocument9 pagesHaemoglobin Acts As Acid Base SystemAli Abbas AslamNo ratings yet
- ACID BASE BALANCE and disordersDocument47 pagesACID BASE BALANCE and disordersShivanand MaliNo ratings yet
- Understanding The PancreasDocument10 pagesUnderstanding The PancreasijostlNo ratings yet
- Chapter 1 Acid-Base Regulation: Lesser Amounts of Organic Acid Derive From The FollowingDocument52 pagesChapter 1 Acid-Base Regulation: Lesser Amounts of Organic Acid Derive From The FollowingMarwa RagabNo ratings yet
- Lec 14 - Kidney PDFDocument16 pagesLec 14 - Kidney PDFrajeshNo ratings yet
- Chapter-7 Acid Base Regulation I) Blood Hydrogen Ion Concentration Is Normally Maintained Around Normal Value ofDocument33 pagesChapter-7 Acid Base Regulation I) Blood Hydrogen Ion Concentration Is Normally Maintained Around Normal Value ofPukhtoon ZalmyNo ratings yet
- Allegato 3 - British Journal of Anaesthesia 1991 - 67 - 154-167Document11 pagesAllegato 3 - British Journal of Anaesthesia 1991 - 67 - 154-167Paolo FassinaNo ratings yet
- Acid Base and Respiratory PhysiologyDocument45 pagesAcid Base and Respiratory PhysiologyTee Sze WayNo ratings yet
- REGULATION OF ACID-BASE BALANCE BY THE KIDNEYSDocument1 pageREGULATION OF ACID-BASE BALANCE BY THE KIDNEYSSatyajitNo ratings yet
- Pleno - Scenario 3 UrogenitalDocument40 pagesPleno - Scenario 3 UrogenitalkhalidahudahNo ratings yet
- L6 Renal Regulation of Acid-Base Balance 2023Document5 pagesL6 Renal Regulation of Acid-Base Balance 2023bgj9cddvxhNo ratings yet
- Role of The KidneysDocument3 pagesRole of The KidneystidesenNo ratings yet
- GINJAL DALAM KESETIMBANGAN ASAM BASA DARAHDocument30 pagesGINJAL DALAM KESETIMBANGAN ASAM BASA DARAHgardamdNo ratings yet
- Buffer Systems in The Body: Protein Buffers in Blood Plasma and CellsDocument11 pagesBuffer Systems in The Body: Protein Buffers in Blood Plasma and CellsK Jayakumar KandasamyNo ratings yet
- Chapter 2 Electrolytes and Body Fluid AnalysiDocument103 pagesChapter 2 Electrolytes and Body Fluid AnalysiSanyii MamuyeNo ratings yet
- A2 OCR Biology - ExcretionDocument3 pagesA2 OCR Biology - ExcretionMokbel ZakariaNo ratings yet
- Acid Secretion by The KidneyDocument37 pagesAcid Secretion by The KidneyHakimah K. Suhaimi100% (1)
- Autonomous State Medical College Shahjahanpur, Uttar Pradesh AnantDocument17 pagesAutonomous State Medical College Shahjahanpur, Uttar Pradesh Anantrahul dev varunNo ratings yet
- Physiology Lec 3Document8 pagesPhysiology Lec 3allid2872No ratings yet
- Acid Base ImbalancesDocument7 pagesAcid Base ImbalancesNicholas TagleNo ratings yet
- Electrolytes & Acid-Base Homeostasis (Keseimbangan Asam-Basa Dan Elektrolit)Document48 pagesElectrolytes & Acid-Base Homeostasis (Keseimbangan Asam-Basa Dan Elektrolit)Andwitya PrameshwariNo ratings yet
- ACID BASE II QuestionsDocument1 pageACID BASE II Questionsmarsay.wheelerNo ratings yet
- 08 Acid-Base BalanceDocument12 pages08 Acid-Base BalanceLeonilaEnriquezNo ratings yet
- Biochemistry of Urine Functions and AnalysisDocument29 pagesBiochemistry of Urine Functions and AnalysisAnonymous hTivgzixVNNo ratings yet
- Blood Gases, PH, and Buffer SystemDocument22 pagesBlood Gases, PH, and Buffer SystemAhmed GaberNo ratings yet
- ACID BASE PHYSIOLOGY: A GUIDE TO CHEMICAL BUFFER SYSTEMS AND H+ REGULATIONDocument28 pagesACID BASE PHYSIOLOGY: A GUIDE TO CHEMICAL BUFFER SYSTEMS AND H+ REGULATIONParvathy R NairNo ratings yet
- Rare and Endangered Livestock Breeds Face ExtinctionDocument17 pagesRare and Endangered Livestock Breeds Face ExtinctionPrakash PanthiNo ratings yet
- Acute Inflammation PDFDocument18 pagesAcute Inflammation PDFJessica Bittar CamargoNo ratings yet
- Cellular Pathology: Normal CellsDocument22 pagesCellular Pathology: Normal CellsPrakash PanthiNo ratings yet
- Animal Breeding - Lecture 1 To 4Document114 pagesAnimal Breeding - Lecture 1 To 4Prakash PanthiNo ratings yet
- Disorders of Cell Growth & NeoplasiaDocument62 pagesDisorders of Cell Growth & NeoplasiaPrakash PanthiNo ratings yet
- Introduction To Pathology: by Gandi Li Department of Pathology West China Medical School Feb, 2003Document72 pagesIntroduction To Pathology: by Gandi Li Department of Pathology West China Medical School Feb, 2003Prakash PanthiNo ratings yet
- PM Artifacts WEBDocument20 pagesPM Artifacts WEBSalman MemonNo ratings yet
- Lec10.1Bacterial Growth CurveDocument8 pagesLec10.1Bacterial Growth CurvePrakash PanthiNo ratings yet
- Physiology o F GustationDocument26 pagesPhysiology o F GustationPrakash PanthiNo ratings yet
- Physiology of Skin FunctionDocument50 pagesPhysiology of Skin FunctionPrakash PanthiNo ratings yet
- Physiology of VisionDocument49 pagesPhysiology of VisionPrakash PanthiNo ratings yet
- Question BankDocument63 pagesQuestion BankPrakash PanthiNo ratings yet
- ThrombosisDocument48 pagesThrombosisPrakash PanthiNo ratings yet
- Namaste and Wel-Come!: Basnet, Hom BahadurDocument26 pagesNamaste and Wel-Come!: Basnet, Hom BahadurPrakash PanthiNo ratings yet
- Excretory SystemDocument49 pagesExcretory SystemPrakash PanthiNo ratings yet
- Physiology of vomiting and defecation: a concise overviewDocument23 pagesPhysiology of vomiting and defecation: a concise overviewPrakash PanthiNo ratings yet
- 4 SalivaDocument93 pages4 SalivaPrakash PanthiNo ratings yet
- Urine FormationDocument76 pagesUrine FormationPrakash PanthiNo ratings yet
- Physiology of HearingDocument36 pagesPhysiology of HearingPrakash PanthiNo ratings yet
- Physiology of vomiting and defecation: a concise overviewDocument23 pagesPhysiology of vomiting and defecation: a concise overviewPrakash PanthiNo ratings yet
- Introduction to gastrointestinal motilityDocument29 pagesIntroduction to gastrointestinal motilityPrakash PanthiNo ratings yet
- Digestion in RuminantsDocument59 pagesDigestion in RuminantsPrakash PanthiNo ratings yet
- Excretory System of BirdDocument20 pagesExcretory System of BirdPrakash PanthiNo ratings yet
- Functional Anatomy of Digestive TractDocument106 pagesFunctional Anatomy of Digestive TractPrakash PanthiNo ratings yet
- Digestive Physiology of Farm Animals: WF-R Animal Science 1 WF-R Animal Science 1Document41 pagesDigestive Physiology of Farm Animals: WF-R Animal Science 1 WF-R Animal Science 1Prakash PanthiNo ratings yet
- Hormone Regulation & Endocrine StructuresDocument28 pagesHormone Regulation & Endocrine StructuresFujoshiNo ratings yet
- Anatomy and Physiology OverviewDocument19 pagesAnatomy and Physiology OverviewRitakamal Bin Mohamad YunusNo ratings yet
- Book Reviews: Halophilic MicroorganismsDocument2 pagesBook Reviews: Halophilic MicroorganismsAlejndrNo ratings yet
- HTTP WWW - Faithfreedom.org Book PDFDocument1 pageHTTP WWW - Faithfreedom.org Book PDFTyas JuniartoNo ratings yet
- AcceptedDocument28 pagesAcceptedChistian LassoNo ratings yet
- Ra 6969Document86 pagesRa 6969Kit BontilaoNo ratings yet
- Drug Education, Prevention and ControlDocument18 pagesDrug Education, Prevention and ControlMaurene MendozaNo ratings yet
- HOAXESDocument2 pagesHOAXESRovelyn S DupraNo ratings yet
- Heart Disease PredictionDocument16 pagesHeart Disease PredictionTarannum ShaikNo ratings yet
- Lecture18 PDFDocument13 pagesLecture18 PDFRyanNugrahaPranantaNo ratings yet
- Group 8Document14 pagesGroup 8HrishavNo ratings yet
- English practice test with 20 questionsDocument23 pagesEnglish practice test with 20 questionsHà ChiNo ratings yet
- Ejercicio de Vocabulario Sobre Partes Del Cuerpo y EnfermedadesDocument5 pagesEjercicio de Vocabulario Sobre Partes Del Cuerpo y EnfermedadesSandra RestrepoNo ratings yet
- Uterin InversionDocument8 pagesUterin InversionZahra AlSaif100% (1)
- Poster of SeptilinDocument1 pagePoster of SeptilinNishtha KumarNo ratings yet
- 50 Pediatric Anesthesia Questions: © 2004, Roy G. Soto, M.DDocument4 pages50 Pediatric Anesthesia Questions: © 2004, Roy G. Soto, M.DGanda SilitongaNo ratings yet
- Four Scenarios for the Future of BioSciences Report HighlightsDocument8 pagesFour Scenarios for the Future of BioSciences Report HighlightsTejaNo ratings yet
- Breast CancersDocument9 pagesBreast CancersMS AntikaNo ratings yet
- Expanding The Therapeutic Window For Acute Ischemic Stroke:: New Agents, New ApproachesDocument29 pagesExpanding The Therapeutic Window For Acute Ischemic Stroke:: New Agents, New ApproachesDr. RajibNo ratings yet
- Dengue Hemorrhagic FeverDocument54 pagesDengue Hemorrhagic FeverfortuneayaNo ratings yet
- LILLE FRANCE Trip Report-MajaliwaDocument4 pagesLILLE FRANCE Trip Report-MajaliwaMajaliwa TungarazaNo ratings yet
- Insulin MinDocument23 pagesInsulin MinLukasNo ratings yet
- Frozen Fresh Food As One of The Solutions To Help Isolated Victims of Disaster Get Healthy FoodDocument52 pagesFrozen Fresh Food As One of The Solutions To Help Isolated Victims of Disaster Get Healthy FoodB1Riansyah FakhiratunnisaNo ratings yet
- (Bioethics 2) 2.02.01 - Case - Kimberly Bergalis (Pat G)Document3 pages(Bioethics 2) 2.02.01 - Case - Kimberly Bergalis (Pat G)Asylum AllegoryNo ratings yet
- Kelsey Camangian: August 2019 - PresentDocument1 pageKelsey Camangian: August 2019 - Presentapi-583563266No ratings yet
- Drug StudyDocument1 pageDrug StudyTHERESE YZABEL CLOMANo ratings yet
- Hair NailsDocument4 pagesHair Nailsapi-26570979No ratings yet