Professional Documents
Culture Documents
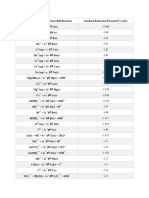
Electrochemical Series of Elements
Uploaded by
rashaCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Electrochemical Series of Elements
Uploaded by
rashaCopyright:
Available Formats
The Electrochemical Series of Elements:
The elements are ordered according to their standard
electrode potential as shown in next table. Elements
with less potential are more active or less noble and
can displace other metals in their solutions. Whereas
metals with more potentials are less active or more
noble i.e., Zn (-0.76) can displace iron.
(-0.44) and also Mg (-2.37) can displace Zn.
Table of Standard emf series of metals
Metal- metal Electrode potential
ion equilibrium At 25° C, volts
Au – Au+3 +1.498
Pt –Pt+2 +1.2
Noble or
Pd –Pd+2 +0.987
Cathodic Ag –Ag+ +0.799
Hg –Hg+2 +0.788
Cu –Cu+2 +0.337
H –H+ 0.000
Pb- Pb+2 -0.126
Sn- Sn+2 -0.136
Ni –Ni+2 -0.250
Co- Co+2 -0.277
Cd- Cd+2 -0.408
Active or
Fe- Fe+2 -0.440
anodic Cr- Cr+3 -0.744
Zn- Zn+2 -0.763
Al- Al+3 -1.662
Mg- Mg+2 -2.363
Na- Na+ -2.714
K- K+ -2.925
You might also like
- Electrochemical Series - CRC Handbook of Chemistry and PhysicsDocument11 pagesElectrochemical Series - CRC Handbook of Chemistry and Physicsmiguel reynagaNo ratings yet
- Standard Redox Potential Table PDFDocument10 pagesStandard Redox Potential Table PDFFercho LotudoNo ratings yet
- Electrochemical EnergyDocument50 pagesElectrochemical EnergyDanica BalmeoNo ratings yet
- Electrochemical SeriesDocument9 pagesElectrochemical Serieszeshma iqbalNo ratings yet
- Electrochemistry Revision-3 OnlineDocument10 pagesElectrochemistry Revision-3 Onlinetumimogotsi14No ratings yet
- High School Science - Redox ReactionsDocument12 pagesHigh School Science - Redox ReactionsPort of Long BeachNo ratings yet
- Electrochemistry GR 12 - TheoryDocument45 pagesElectrochemistry GR 12 - TheoryPepsiNo ratings yet
- Standard Reduction PotentialsDocument1 pageStandard Reduction PotentialsCamiloNo ratings yet
- Wang Battery and EV PDFDocument101 pagesWang Battery and EV PDFMateo DomínguezNo ratings yet
- Cpe639 Lecture 4Document73 pagesCpe639 Lecture 4Aisyah Addia AzizanNo ratings yet
- Standard Electrode and Reduction Potentials at 298 K PrintableDocument3 pagesStandard Electrode and Reduction Potentials at 298 K Printablecarina_yii9690No ratings yet
- Lecture 21 - Corrosion - July 19Document14 pagesLecture 21 - Corrosion - July 19Ryan MaxwellNo ratings yet
- Reduction and Oxidation PotentialDocument1 pageReduction and Oxidation Potentialioan_vNo ratings yet
- Elc STD PotentialsDocument1 pageElc STD PotentialsArchita VNo ratings yet
- Corrosion Lecture 1 Material EngineeringDocument14 pagesCorrosion Lecture 1 Material EngineeringBaso Rahmat TarmiziNo ratings yet
- ch18 MSW15 Corrosion PDFDocument41 pagesch18 MSW15 Corrosion PDFMiralda SyakirahNo ratings yet
- Standard Cell Potentials PracticesDocument3 pagesStandard Cell Potentials PracticeservaldiNo ratings yet
- Electro-Chemical SeriesDocument2 pagesElectro-Chemical SeriesS S V Jagannadha Sarma GummaNo ratings yet
- Acid and Base and RedoxDocument53 pagesAcid and Base and RedoxH M AwaisNo ratings yet
- Standard Reduction PotentialsDocument3 pagesStandard Reduction PotentialsjaverfrivNo ratings yet
- Standard Reduction PotentialsDocument5 pagesStandard Reduction PotentialsnathaloaNo ratings yet
- P2 Standard Reduction Potentials by ValueDocument6 pagesP2 Standard Reduction Potentials by ValueASTRID ELIZABET CUEVA GUTIERREZNo ratings yet
- Standard Potentials at 298 K. (A) in Electrochemical Order: Table 6.2Document2 pagesStandard Potentials at 298 K. (A) in Electrochemical Order: Table 6.2Alexander RodriguezNo ratings yet
- Application of Electrolytic Cells Lesson 11Document24 pagesApplication of Electrolytic Cells Lesson 11Rosmaini MohamadNo ratings yet
- Chapter 4 ClassDocument64 pagesChapter 4 ClassWai Myo HtunNo ratings yet
- E ValuesDocument1 pageE ValuesShania LoveresNo ratings yet
- 9.12 Electrochemistry Half Reactions IntroDocument5 pages9.12 Electrochemistry Half Reactions IntroPatrick AbidraNo ratings yet
- Standard Calulation For Potentials AcrossDocument2 pagesStandard Calulation For Potentials Acrossmurugan_kribhcoNo ratings yet
- Tabla Potencial Reduccion PDFDocument13 pagesTabla Potencial Reduccion PDFFóxel ArgNo ratings yet
- 12 DChem Research SolubilityDocument6 pages12 DChem Research SolubilityRenzelle MelisseNo ratings yet
- Tabla de PotencialesDocument6 pagesTabla de PotencialesLuis AntonioNo ratings yet
- Chemistry Data Sheet: Formulae of Common Ions Reactivity Series Positive Negative Elements ReactivityDocument1 pageChemistry Data Sheet: Formulae of Common Ions Reactivity Series Positive Negative Elements ReactivityIsrael PopeNo ratings yet
- Chemistry Data Sheet: Formulae of Common Ions Reactivity Series Positive Negative Elements ReactivityDocument1 pageChemistry Data Sheet: Formulae of Common Ions Reactivity Series Positive Negative Elements ReactivityIsrael PopeNo ratings yet
- 1corrosion and DegradationDocument12 pages1corrosion and DegradationParyanto Dwi SetyawanNo ratings yet
- Corrosion and Degradation of Materials: Issues To Address..Document12 pagesCorrosion and Degradation of Materials: Issues To Address..Pankaj Kumar SainiNo ratings yet
- Tabla de Potenciales Redox PDFDocument14 pagesTabla de Potenciales Redox PDFAna Altamirano100% (1)
- Corrosion & DegradationDocument36 pagesCorrosion & Degradationrenan.masangya-18No ratings yet
- Serie ElectroquímicaDocument10 pagesSerie ElectroquímicaÁngeles LópezNo ratings yet
- Standard Reduction PotentialDocument7 pagesStandard Reduction Potentialyoyotoonzone1No ratings yet
- Potenciales Estandar Del ElectrodoDocument3 pagesPotenciales Estandar Del ElectrododavidNo ratings yet
- ElectrolysisDocument46 pagesElectrolysisHelen WangNo ratings yet
- Electrochemistry 1Document74 pagesElectrochemistry 1Vipranshu GuptaNo ratings yet
- Oxidation and Reduction Workbook Revised 1ADocument15 pagesOxidation and Reduction Workbook Revised 1AMarisa St. LouisNo ratings yet
- Redox Reaction: Chem 16 Lab Second Long ExamDocument4 pagesRedox Reaction: Chem 16 Lab Second Long ExamAmethyst GomezNo ratings yet
- EMF SeriesDocument5 pagesEMF Seriesmike rosaNo ratings yet
- Potencial EletroquimicoDocument13 pagesPotencial EletroquimicoMatheus EduardoNo ratings yet
- Material Science: Prof. Satish V. KailasDocument10 pagesMaterial Science: Prof. Satish V. KailasHagere EthiopiaNo ratings yet
- Standard Reduction Potentials at 25Document1 pageStandard Reduction Potentials at 25Beverly RamosNo ratings yet
- E41ad 9d85Document18 pagesE41ad 9d85sayyed bassir ajellehNo ratings yet
- 78 128Document51 pages78 128Anonymous qKeDFDNo ratings yet
- E-EMM 3122-9-Corrosion and Protection (N)Document13 pagesE-EMM 3122-9-Corrosion and Protection (N)KHAIRUL NASHRAN BIN ANUAR / UPMNo ratings yet
- Worksheet 01 Dated 05072021Document1 pageWorksheet 01 Dated 05072021Jeny SharmaNo ratings yet
- MST 2017 eDocument2 pagesMST 2017 eNavnoor kaurNo ratings yet
- Electrochemistry 13 THDocument36 pagesElectrochemistry 13 THRaju SinghNo ratings yet
- Kims CopiesDocument17 pagesKims Copieszafarchem_iqbalNo ratings yet
- Adobe Scan Nov 19, 2021Document1 pageAdobe Scan Nov 19, 2021Diana FrancisNo ratings yet
- Born Haber CycleDocument2 pagesBorn Haber CycleKhalid Ibrahim PatelNo ratings yet