Professional Documents
Culture Documents
Identification of Crystal Types Lab Nick Diamantakos
Uploaded by
Nick DiamantakosOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Identification of Crystal Types Lab Nick Diamantakos
Uploaded by
Nick DiamantakosCopyright:
Available Formats
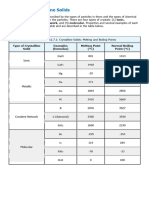
IDENTIFICATION OF CRYSTAL TYPES LAB NICK DIAMANTAKOS
Does it dissolve in water?
Yes
No
Does the solution conduct electricity? No
Does it dissolve in mineral oil?
Yes
Yes
No
Is it ductile, and malleable? Ionic Crystal Molecular Crystal - Polar -Polar Molecules -Electrostatic Attractions -High melting points -High conductivity -Hard, brittle -Dissolve in polar solutions -Between atoms with different electro negativities -Electrostatic attractions between dipoles -May have hydrogen bonding -Atoms or non-polar molecules -LDF forces -Low melting point -Extremely low conductivity -Very soft -Soluble in nonpolar solvents Metal -Atoms or non-polar molecules -LDF forces -Low melting point Covalent Network -Atoms -Covalent Bonds Molecular Crystal NonPolar
Yes
NO
-Positive and negative ions
-Intermediate melting points
-Low conductivity -Fragile, weak IMF -Soluble in Polar
-Very high
-Extremely low conductivity -Very soft -Very hard -Soluble in non-polar solvents -Insoluble -Low conductivity
You might also like
- El Chuletatorbonds - Programa Online Creador de Chuletas para Copiar en ExámenesDocument1 pageEl Chuletatorbonds - Programa Online Creador de Chuletas para Copiar en ExámenesAdrián GarcíaNo ratings yet
- Bonding Prelab Answer KeyDocument1 pageBonding Prelab Answer KeyRosebud ThiriNo ratings yet
- Summary of Bonding Elements Compounds Intermolecular Forces PropertiesDocument1 pageSummary of Bonding Elements Compounds Intermolecular Forces PropertiesChirag HablaniNo ratings yet
- Covalent Network MoleculesDocument1 pageCovalent Network MoleculesGill CraigNo ratings yet
- Chemistry Lab Unit 1 - MurtazaDocument3 pagesChemistry Lab Unit 1 - MurtazaMurtaza hussainNo ratings yet
- Solid State 6 JulyDocument18 pagesSolid State 6 JulyQwertyNo ratings yet
- 4.5 Physical Properties: Syllabus StatementsDocument1 page4.5 Physical Properties: Syllabus StatementsBreeSchuchNo ratings yet
- Solnform PresDocument34 pagesSolnform PresMycah LongboyNo ratings yet
- Chemistry Ionic LatticeDocument11 pagesChemistry Ionic LatticeJunKenNo ratings yet
- (Junoon-E-Jee 3.0) Solid StateDocument119 pages(Junoon-E-Jee 3.0) Solid StateShiven DhaniaNo ratings yet
- The Nature of SolidsDocument11 pagesThe Nature of SolidsnsuperticiosoNo ratings yet
- PT TrendsandpropertiesDocument49 pagesPT TrendsandpropertiesshizukesakeitoNo ratings yet
- Chem M1 PDFDocument11 pagesChem M1 PDFZarylle De AsasNo ratings yet
- PDF Document 5Document25 pagesPDF Document 5miriam harriottNo ratings yet
- Covalent and Ionic Properties LabDocument6 pagesCovalent and Ionic Properties LabMadi WellsNo ratings yet
- Part 2 Acid Base Metal Non MetalDocument35 pagesPart 2 Acid Base Metal Non MetalCay C. CordovaNo ratings yet
- COLLOIDSDocument8 pagesCOLLOIDSJanna Kate SajullaNo ratings yet
- Solution and SolubilityDocument64 pagesSolution and SolubilitySohila A. MabroukNo ratings yet
- States of Matter - Solids, Liquids, Gases & Plasma - ChemistryDocument8 pagesStates of Matter - Solids, Liquids, Gases & Plasma - Chemistryjerikbenito46No ratings yet
- The Solid State: Chapter - 15Document16 pagesThe Solid State: Chapter - 15Athish MNo ratings yet
- Too Share or Not To ShareDocument6 pagesToo Share or Not To ShareSpencer JorgensenNo ratings yet
- Unidad 61Document36 pagesUnidad 61Eloisa OvandoNo ratings yet
- Chemistry Test 5 Study GuideDocument3 pagesChemistry Test 5 Study GuideLeanne RoseNo ratings yet
- Classification of Crystaline Solids-Sneha LathaDocument18 pagesClassification of Crystaline Solids-Sneha LathaTarun YadavNo ratings yet
- Periodic Table (Chemical Bonding)Document8 pagesPeriodic Table (Chemical Bonding)Teresa Marie CorderoNo ratings yet
- The Properties of Ionic and Covalent Compounds: Experiment 4Document5 pagesThe Properties of Ionic and Covalent Compounds: Experiment 4MUHAMMAD AKRAMNo ratings yet
- Summary of Bonding, Structure and Properties of SubstancesDocument3 pagesSummary of Bonding, Structure and Properties of SubstancesAnonymous L7ZuSkR100% (1)
- Chemistry HWDocument5 pagesChemistry HWh9gfvyjr8gNo ratings yet
- Unit 3 Solutions POWERPOINT 3Document81 pagesUnit 3 Solutions POWERPOINT 3Jenny YoonNo ratings yet
- Properties of Liquids and SolidsDocument33 pagesProperties of Liquids and SolidsNicolette BingtanNo ratings yet
- CHM ReportingDocument70 pagesCHM ReportingNica Rose GrozenNo ratings yet
- Intermolecular Forces (Presentation)Document22 pagesIntermolecular Forces (Presentation)Rianne JusainNo ratings yet
- Chemistry Sec 3 NotesDocument17 pagesChemistry Sec 3 NotesChua Zong Han50% (4)
- Gpiv PDFDocument4 pagesGpiv PDFSamson AmosNo ratings yet
- LAB #2-Ionic and CovalentDocument3 pagesLAB #2-Ionic and CovalentshadowNo ratings yet
- Q3 Module 2A - Nature of Solids and Phase Changes 1Document34 pagesQ3 Module 2A - Nature of Solids and Phase Changes 1Rance BobadillaNo ratings yet
- Phisycal Chemistry SolsDocument28 pagesPhisycal Chemistry SolsAdilla Rizka YonitaNo ratings yet
- Share 'S-BLOCK ELEMENTSDocument33 pagesShare 'S-BLOCK ELEMENTSAyush Kumar Bhaladhare.115No ratings yet
- Types of BondingDocument7 pagesTypes of Bondingukpics7No ratings yet
- Classes of Crystalline Solids 4 Types of CrystalDocument5 pagesClasses of Crystalline Solids 4 Types of Crystaldellrubion011No ratings yet
- Case Studies in Bonding and StructureDocument3 pagesCase Studies in Bonding and StructureDoc_CrocNo ratings yet
- Physical Chemistry 2 - Thermodynamics of Electrolyte Solutions - v3Document63 pagesPhysical Chemistry 2 - Thermodynamics of Electrolyte Solutions - v3Nguyễn Thu HàNo ratings yet
- GenChem2 3Document25 pagesGenChem2 3Jan LagriaNo ratings yet
- Bonding & Molecular Structure: Topic Outline: Directions For BLOOMS - Lower Order - UnderstandingDocument4 pagesBonding & Molecular Structure: Topic Outline: Directions For BLOOMS - Lower Order - Understandingapi-320784618No ratings yet
- Lesson5 - Structure of Crystalline and Amorphous LiquidsDocument19 pagesLesson5 - Structure of Crystalline and Amorphous LiquidsLemonadeNo ratings yet
- GCE Singapore-Cambridge Pure Chemistry NotesDocument21 pagesGCE Singapore-Cambridge Pure Chemistry NotesChong56No ratings yet
- Kinetic Molecular Model of Solids and LiquidsDocument36 pagesKinetic Molecular Model of Solids and LiquidsYard BirdNo ratings yet
- Solid Crystalline, Amorphous & Polymorphism PDFDocument33 pagesSolid Crystalline, Amorphous & Polymorphism PDFPrabhas Meher100% (1)
- The Solid State PDFDocument69 pagesThe Solid State PDFVNS TechNo ratings yet
- The Solid State ModifiedDocument70 pagesThe Solid State ModifiedR220603 THAMMINENI MALLIKARJUNANo ratings yet
- Unit V Solutions: Solution ChemistryDocument7 pagesUnit V Solutions: Solution ChemistryCharlie SobcovNo ratings yet
- Chapter 5 - SolidDocument27 pagesChapter 5 - SolidLooshani MariappanNo ratings yet
- Role of Solvent in SNDocument9 pagesRole of Solvent in SNsarahNo ratings yet
- Physical PropertyDocument1 pagePhysical PropertyheenalvNo ratings yet
- HSABDocument3 pagesHSABNasser Al-RiyamiNo ratings yet
- Topic 4 Structure and Properties of Covalent CompoundsDocument3 pagesTopic 4 Structure and Properties of Covalent CompoundsShirleenNo ratings yet
- Science 6: 1 Quarter: MATTERDocument60 pagesScience 6: 1 Quarter: MATTERRichard AlboroNo ratings yet
- Intermolecular and Intramolecular ForcesDocument19 pagesIntermolecular and Intramolecular Forceshazza82005No ratings yet
- Identification and Test For Reagents Planet Earth: Atomic Structure Relative Isotopic, Atomic and Molecular MassesDocument8 pagesIdentification and Test For Reagents Planet Earth: Atomic Structure Relative Isotopic, Atomic and Molecular MassesTSZ YAN CHEUNGNo ratings yet