Professional Documents
Culture Documents
73119482 Tổng quat giản đồ Frost
Uploaded by
boyhohoOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
73119482 Tổng quat giản đồ Frost
Uploaded by
boyhohoCopyright:
Available Formats
Tng qut gin Frost: Gin Frost c dng trong in ho m t mi tng quan bn ca cc trng thi oxi ho khc nhau
nhau ca cht nn (oxidation state diagram). Mi gin s tng ng vi mt gi tr pH nht nh, v ng vi mi gi tr pH th cc thng s bn ca tng trng thi cht nn s khc nhau. C th ni, gin Frost thc ra l bn (version) Latimer hai chiu (2D) vi s thm vo thng s s electron trao i.
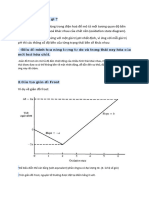
Hnh trn m t m hnh chung nht ca gin Frost, tng ng vi h sau: M(II) + 2e --> M (0) ; E- = -a M(VI) + 4e --> M (II) ; E- = b Vic s dng gin ny s hu dng trong vic hnh dung kh nng, tin trnh nhng phn ng c hng s cn bng ln hn n v hoc mang mt gi tr khc kh phn on, m ta ko cn thit phi qua tnh ton. Nh ta bit, trong tng bn phn ng, mi electron trao i s mang theo mt gi tr nng lng t do xc nh, tun theo cng thc sau: G = -nFE Do vy, biu din th cn bng (volt-equivalent) phn ng, ta c mt i lng hp l l nE-, trong , E- l dc ca ng biu din (xem hnh). Trn gin Frost, nguyn t thng c t ti im chun bng 0 volt. im cng nm bn di trong gin Frost th c trng h thng ti im cng bn. T cho ta xc nh c trng thi oxi ho bn nht ca nguyn t. Nhng im nm pha trn bn phi trong gin Frost c trng cho nguyn t trong h s phn ng mnh lit vi tc nhn oxi ho mnh (cht kh mnh). Bng vic phn tch thng s trn gin , ta c th ch ra c kh nhiu tnh cht (properties), mi tng quan cng nh kh nng chuyn ho (bng phn ng) qua li
gia cc trng thi oxi ho khc nhau ca cht nn. Gin Frost c th c xy dng t gin Latimer, ch cn bit s electron trao i trong mi bn phn ng. Gi tr nm trn trc y ca gin thu c bng cch nhn s lng electron dch chuyn khi thay i trng thi oxi ho vi s thay i th kh chun, hoc nng lng t do (F). Chng hn nh vi gin Latimer ca Vanadi trong dung dch acid nh sau:
Ta c th xy dng gin Frost:
Lu i vi nhng trng thi oxi ho ca Vanadium, mt c trng l trong dung dch acid, trng thi s oxi ho +3 chim u th, trong khi xt v mt kh nng xy ra phn ng t oxi ho - kh (s oxi ho bn) th hai trng thi +2 vi +4 cng bn. r hn, ta xt gin Frost ca Mn lm v d:
Nhng thng tin g c th bit c v ko th bit c t gin Frost: + bn nhit ng i vi nhng trng thi bn di ca gin . Tht vy, nm v tr cng thp trong gin , bn nhit ng cng cao. Nh gin trn, Mn (II) l bn nht v mt nhit ng. + Nhng hnh thi trn vng li ca ng biu din thng km bn, c th thc hin s d phn. Nh MnO42- v Mn (III) c khuynh hng xy ra phn ng d phn (disproportionation reaction). + Nhng hnh thi trn vng lm ca ng biu din khng b d phn, tng ng vi trng thi oxi ho bn. Nh MnO2 l mt trng thi oxi ho bn. C th m hnh ho gin hai im trn nh sau:
gin (a) tng ng vi trng thi phn ng oxi ho - kh kt hp (proportionation), ngc li gin (b) tng ng trng thi phn ng t oxi ho - kh hay d phn (disproportionation). Trng thi C (species) gin (a) tng ng vng lm, th hin trng thi oxi ho bn; trong khi trng thi C gin (b) tng ng vng li, th hin trng thi oxi ho km bn. + Nhng hnh thi cng nm v pha trn bn tri ca gin s l tc nhn oxi ho mnh. MnO4- l mt cht oxi ho mnh. + Nhng hnh thi cng nm v pha trn bn phi ca gin s l tc nhn kh mnh. Mn kim loi l mt cht kh va phi. + Gin Frost m t bn nhit ng ca nhiu hnh thi khc nhau, mc d c nhng hnh thi c a ra c th khng bn v mt nhit ng, d dn n s kh, ng hc ca cc phn ng ny rt chm. V d: Mc d yu t nhit ng lc hc ca s kh ion permanganate MnO42- v Mn(II) l u tin, nhng phn ng xy ra chm nu ko c s tham gia ca xc tc. Tht vy, dung dch permanganate c th c bo qun v dng trong phng th nghim. + Thng tin thu c t gin Frost i vi mi hnh thi di iu kin chun (pH = 0 i vi dung dch acid v pH = 14 i vi dung dch base). Thay i pH c th lm thay i bn tng i ca cc hnh thi. Th ca nhiu qu trnh bao gm ion hydrogen s thay i vi pH v nng ca hnh thi thay i. V d: Di iu kin base, dung dch Mn2+ khng tn ti, thay vo l trng thi kt ta Mn(OH)2.
You might also like
- Lý thuyết pin điện PDFDocument6 pagesLý thuyết pin điện PDFHiếu VõNo ratings yet
- Thế điện cực và suất điện động của pin điện PDFDocument23 pagesThế điện cực và suất điện động của pin điện PDFLong Nguyenduy25% (4)
- Bốn khám phá Căn bản Đặc biệt quan trọng cho Hóa học: Four basic Discoveries Especially Important for ChemistryFrom EverandBốn khám phá Căn bản Đặc biệt quan trọng cho Hóa học: Four basic Discoveries Especially Important for ChemistryNo ratings yet
- Phuc ChatDocument14 pagesPhuc ChatBich VanNo ratings yet
- Nhom Iia-Iib PDFDocument42 pagesNhom Iia-Iib PDFnam namNo ratings yet
- de Thi - Olympic SV-2016 - Bang BDocument3 pagesde Thi - Olympic SV-2016 - Bang BTranggNo ratings yet
- Tiểu Luận - Cơ Chế Phản Ứng Electrophin Trong Liên Kết Cacbon-cacbon Của Anken Và Ankin - 369273Document14 pagesTiểu Luận - Cơ Chế Phản Ứng Electrophin Trong Liên Kết Cacbon-cacbon Của Anken Và Ankin - 369273Quoc AnhNo ratings yet
- Chương 3. Một Số Tính Chất Của Phức ChấtDocument14 pagesChương 3. Một Số Tính Chất Của Phức ChấtPhượng NguyễnNo ratings yet
- 1. Hiệu ứng co d - co fDocument6 pages1. Hiệu ứng co d - co fBùi Thị Thanh HàNo ratings yet
- Chương 4. Dai Cuong NTCT G I SVDocument9 pagesChương 4. Dai Cuong NTCT G I SVPhượng NguyễnNo ratings yet
- Đề Cương Bài Giảng: Học Phần: Hóa Lí 3 - Điện Hóa HọcDocument108 pagesĐề Cương Bài Giảng: Học Phần: Hóa Lí 3 - Điện Hóa HọcLÊ THỊ HẰNGNo ratings yet
- Nito VIDocument22 pagesNito VIVân Trần Thu0% (1)
- So sánh và giải thích độ mạnhDocument10 pagesSo sánh và giải thích độ mạnhGia Sư Hóa HọcNo ratings yet
- (123doc) - Bai-Tap-Hoa-Vo-Co-Co-Loi-GiaiDocument58 pages(123doc) - Bai-Tap-Hoa-Vo-Co-Co-Loi-GiaiTô Hoàng Thuỳ DungNo ratings yet
- Tiểu Luận Hóa Hữu CơDocument22 pagesTiểu Luận Hóa Hữu CơTram VoNo ratings yet
- C là nồng độ lúc sau, c là nồng độ đầuDocument12 pagesC là nồng độ lúc sau, c là nồng độ đầuTrang NguyễnNo ratings yet
- DFDocument13 pagesDFtrang_lp100% (1)
- Coche2 Chuong-1 PDFDocument46 pagesCoche2 Chuong-1 PDFThư YJsNo ratings yet
- 8.10H-Pin Điện HóaDocument17 pages8.10H-Pin Điện HóaNguyễn Tú 10HNo ratings yet
- hợp chất cơ kimDocument28 pageshợp chất cơ kimtungdajzaNo ratings yet
- Bài tập hóa lýDocument60 pagesBài tập hóa lýJoshep Petrus Copper50% (2)
- Hat NhanDocument15 pagesHat NhanTrang NguyễnNo ratings yet
- PH CDocument74 pagesPH CUyên PhạmNo ratings yet
- Bai Tap Pin DienDocument34 pagesBai Tap Pin DienLai NguyênNo ratings yet
- Chuyên đề - Hiệu ứng cấu trúc PDFDocument18 pagesChuyên đề - Hiệu ứng cấu trúc PDFHinhhoc12No ratings yet
- Bài tập lớn Vận dụng thuyết cấu tạo Hoá học để giải một số bài tập định tính phần phi kim trong đề thi học sinh giỏi Hóa học và đề thi Olympic Hóa họcDocument28 pagesBài tập lớn Vận dụng thuyết cấu tạo Hoá học để giải một số bài tập định tính phần phi kim trong đề thi học sinh giỏi Hóa học và đề thi Olympic Hóa họcThắng ĐứccNo ratings yet
- Lai Hoa Obitan Nguyen TuDocument6 pagesLai Hoa Obitan Nguyen Tuan_thvt100% (1)
- Pin Điện HóaDocument312 pagesPin Điện HóaTrần Nghiêm ThànhNo ratings yet
- Ung Dung Cua Gian Do LATIMERDocument9 pagesUng Dung Cua Gian Do LATIMERTrần Quang0% (1)
- HSG Nhiet Hoa HocDocument27 pagesHSG Nhiet Hoa HocGấu MèoNo ratings yet
- Tóm Tắt Lý Thuyết Và Một Số Bài Tập Ôn Thi Hoá Vô CơDocument38 pagesTóm Tắt Lý Thuyết Và Một Số Bài Tập Ôn Thi Hoá Vô CơKing Ken100% (3)
- BÀI TẬP HÓA VÔ CƠ 2022Document13 pagesBÀI TẬP HÓA VÔ CƠ 2022Nguyễn Đức TàiNo ratings yet
- SACH GIAO BAI TAP - HOA LY 2019 Gui SVDocument8 pagesSACH GIAO BAI TAP - HOA LY 2019 Gui SVVũ ĐứcNo ratings yet
- BÀI TẬPDocument12 pagesBÀI TẬPxuyên phạm thịNo ratings yet
- Bài tập Năng lượng ion hóa 1Document3 pagesBài tập Năng lượng ion hóa 1Đinh Hoàng Bích Trâm100% (1)
- Chuong 6. Nguyen To Nhom IIB - CH&BTDocument3 pagesChuong 6. Nguyen To Nhom IIB - CH&BTĐặng Thùy DươngNo ratings yet
- Các dạng bài tập về pin điệnDocument18 pagesCác dạng bài tập về pin điệnnganbao12No ratings yet
- So Sánh Phản Ứng Sn1 Và e1Document3 pagesSo Sánh Phản Ứng Sn1 Và e1Nguyễn Công ChungNo ratings yet
- Các Hợp Chất Đặc Biệt Của Lưu HuỳnhDocument51 pagesCác Hợp Chất Đặc Biệt Của Lưu HuỳnhTrung100% (2)
- Hoa Hoc 11Document11 pagesHoa Hoc 11Đỗ Đình ChiếnNo ratings yet
- Đề Cương Ôn Tập Môn phương pháp dạy học hóa họcDocument2 pagesĐề Cương Ôn Tập Môn phương pháp dạy học hóa họcHà TrangNo ratings yet
- Organic Chemistry Clayden (Dịch) Phần 1Document523 pagesOrganic Chemistry Clayden (Dịch) Phần 117-11H Phan Quang Minh100% (1)
- Phân Biệt Phản Ứng Tách Loại E1 Và E2Document1 pagePhân Biệt Phản Ứng Tách Loại E1 Và E2duyên vũNo ratings yet
- 3.CẤU TRÚC CH. LỰC A-B-1Document4 pages3.CẤU TRÚC CH. LỰC A-B-1Trang NguyễnNo ratings yet
- Vô Cơ 1 - Thầy NgọcDocument259 pagesVô Cơ 1 - Thầy NgọcVăn Tài100% (1)
- CHƯƠNG 2. BT HÓA HỌC ACID BASEDocument4 pagesCHƯƠNG 2. BT HÓA HỌC ACID BASERa Cla67% (3)
- (123doc) - Hoa-11-Cvp-De-Thi-Dap-An-De-Xuat-Trai-He-Hung-Vuong-Moi-NhatDocument10 pages(123doc) - Hoa-11-Cvp-De-Thi-Dap-An-De-Xuat-Trai-He-Hung-Vuong-Moi-NhatQuốc ThắngNo ratings yet
- CHUYÊN ĐỀ NITROGEN - PHOSPHORUS BỒI DƯỠNG HSGDocument55 pagesCHUYÊN ĐỀ NITROGEN - PHOSPHORUS BỒI DƯỠNG HSGLuna Nhung0% (1)
- CẤU TẠO CHẤT - 2Document1 pageCẤU TẠO CHẤT - 2Trang Nguyễn100% (1)
- Dạng 1: Phương pháp so sánh tính Bazo, axit và nhiệt độ sôi của các hợp chất hữu cơDocument4 pagesDạng 1: Phương pháp so sánh tính Bazo, axit và nhiệt độ sôi của các hợp chất hữu cơLê Thị Thảo TrinhNo ratings yet
- P2 Nguyễn Huệ 2021 Phức chấtDocument2 pagesP2 Nguyễn Huệ 2021 Phức chấtHiếu Phan Công100% (1)
- Chương 1 - Những Khái Niệm Cơ Bản Về Phức Chất (Câu 8-11)Document12 pagesChương 1 - Những Khái Niệm Cơ Bản Về Phức Chất (Câu 8-11)Phượng NguyễnNo ratings yet
- Chuyên Đề 2 - Liên Kết Hóa HọcDocument17 pagesChuyên Đề 2 - Liên Kết Hóa HọcPhong Tr100% (1)
- Boọ 30c hóa lí dượcDocument17 pagesBoọ 30c hóa lí dượcPhạm HươngNo ratings yet
- (123doc) - Van-Dung-Ly-Thuyet-Can-Bang-Trong-Dung-Dich-Chua-Hop-Chat-It-Tan-Va-Chuan-Do-Ket-Tua-Trong-Boi-Duong-Hoc-Sinh-Gioi-Thi-Quoc-Gia-Va-Quoc-TeDocument112 pages(123doc) - Van-Dung-Ly-Thuyet-Can-Bang-Trong-Dung-Dich-Chua-Hop-Chat-It-Tan-Va-Chuan-Do-Ket-Tua-Trong-Boi-Duong-Hoc-Sinh-Gioi-Thi-Quoc-Gia-Va-Quoc-TeThảo ThạchNo ratings yet
- Dap An HSG QG 2007 Mon HoaDocument11 pagesDap An HSG QG 2007 Mon Hoafree1550% (2)
- Làm Sao Giải 64 Rubik Với Những Công Thức Đơn GiảnFrom EverandLàm Sao Giải 64 Rubik Với Những Công Thức Đơn GiảnRating: 5 out of 5 stars5/5 (1)
- Tổng quát giản đồ FrostDocument3 pagesTổng quát giản đồ FrostHằng ĐàoNo ratings yet
- dàn ý giản đồ frostDocument4 pagesdàn ý giản đồ frostNam Hóa HọcNo ratings yet
- Bài giảng phân tích điện hóaDocument117 pagesBài giảng phân tích điện hóaNguyen_Bao_6655100% (3)
- 007114 - UD3042 - CQ - Đề kiểm tra Chương 4,5Document5 pages007114 - UD3042 - CQ - Đề kiểm tra Chương 4,5boyhohoNo ratings yet
- Bai Tap Sai SoDocument15 pagesBai Tap Sai SoHồng Nhung Hồng NhungNo ratings yet
- Gioithieu Bo Mon Hoa Vo Co Minh Kha ModifiedDocument21 pagesGioithieu Bo Mon Hoa Vo Co Minh Kha ModifiedboyhohoNo ratings yet
- Thuoc Thu Huu CoDocument290 pagesThuoc Thu Huu CobillytuananhNo ratings yet
- Thuoc Thu Huu CoDocument290 pagesThuoc Thu Huu CobillytuananhNo ratings yet