Professional Documents
Culture Documents
5.60 Thermodynamics & Kinetics: Mit Opencourseware
5.60 Thermodynamics & Kinetics: Mit Opencourseware
Uploaded by
Syed Farhan HassanOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
5.60 Thermodynamics & Kinetics: Mit Opencourseware
5.60 Thermodynamics & Kinetics: Mit Opencourseware
Uploaded by
Syed Farhan HassanCopyright:
Available Formats
MIT OpenCourseWare http://ocw.mit.
edu
5.60 Thermodynamics & Kinetics
Spring 2008
For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms.
5.60 Spring 2008
Lecture #3
page 1
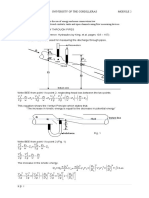
Isothermal Gas Expansion
gas (p1, V1, T) = gas (p2, V2, T)
( T = 0)
Irreversibly (many ways possible) (1) Set pext = 0
p= 0 T p= 0 p 1,V1 T p 2,V2
w (1) = pext dV = 0
V1
v2
(2)
Set pext = p2
p2 T p2 p 1,V1 T p 2,V2
w (2) = p2dV = p2 (V2 V1 )
V1
v2
p p1
p2 V1 V2
-w(2)
Note, work is negative: system expands against surroundings
5.60 Spring 2008
Lecture #3
page 2
(3)
Carry out change in two steps
gas (p1, V1, T) = gas (p3, V3, T) = gas (p2, V2, T)
p3 T p 3,V3
v3 v2
p1 > p3 > p2
p2
p3 p 1,V1
T p 2,V2
w (3) = p3dV p2dV = p3 (V3 V1 ) p2 (V2 V3 )
V1 V3
p p1 p3 p2 V1 V3 V2
More work delivered to surroundings in this case.
-w(3)
(4)
p p1
Reversible change
p = pext throughout
wrev = 2 pdV
V1
p2 V1 V2 rev
Maximum work delivered to surroundings for isothermal gas expansion is obtained using a reversible path
For ideal gas:
wrev = 2
V1
V p nRT dV = nRT ln 2 = nRT ln 2 V V1 p1
5.60 Spring 2008
Lecture #3
page 3
The Internal Energy U -q + d -w dU = d (First Law)
dU = C pathdT pext dV T ,V And U (
U U dU = dT + dV T V V T
Some frequent constraints:
Reversible
-q + d -q pdV -w = d dU = d rev rev rev -w = 0 -q = d d -q = 0 d
(p = pext )
Isolated Adiabatic Constant V
-w reversible dU = d = -pdV
-q w = 0 dU = d V
Constant V
U U dU = dT + dV T V V T but also U -q = d dT V T V
-q = C dT d V V
U = CV T V
very important result!!
So
dU = CV dT +
U V T
dV
what is this?
5.60 Spring 2008
Lecture #3
page 4
Joule Free Expansion of a Gas
(to get
U ) V T
Adiabatic
gas vac
q=0 w=0
gas (p1, T1, V1) = gas (p2, T2, V2) Since q = w = 0 Recall
Expansion into Vac. (pext=0)
dU or U = 0
Constant U
dU = CV dT +
U
V T
U dV = 0 V T
dVU = CV dTU
T V U measure in Joule exp't! T V U
U = CV V T
Joule did this.
lim V 0
T T dU = CV dT CV J dV = J V U V U Joule coefficient
For Ideal gas
U(T)
dU = CV dT
J = 0
exactly Always for ideal gas only depends on T
The internal energy of an ideal gas depends only on temperature
Consequences
U = 0
For all isothermal expansions or compressions of ideal gases
U = CV dT
For any ideal gas change in state
You might also like
- Reading List For Those Interested in The Orthodox ChurchDocument7 pagesReading List For Those Interested in The Orthodox ChurchAlexander Buterbaugh60% (5)
- Solution Manual for an Introduction to Equilibrium ThermodynamicsFrom EverandSolution Manual for an Introduction to Equilibrium ThermodynamicsNo ratings yet
- Hydro 1 Module 2Document13 pagesHydro 1 Module 2Jericho Alfred Rullog Sapitula100% (1)
- Saas Sales Models PDFDocument15 pagesSaas Sales Models PDFgsrrs100% (1)
- Apostolic AgeDocument6 pagesApostolic AgeMINCOR SS CC100% (1)
- A V P A V P: PressureDocument2 pagesA V P A V P: PressurePearl Alexandra FabitoNo ratings yet
- TCCC PDFDocument86 pagesTCCC PDFJaime Acevedo100% (1)
- Chapter 2 FormulasDocument6 pagesChapter 2 FormulasShellyNo ratings yet
- Chapter 34 Exploration and Evaluation of Mineral ResourcesDocument2 pagesChapter 34 Exploration and Evaluation of Mineral ResourcesEllen MaskariñoNo ratings yet
- Investigatory ProjectDocument13 pagesInvestigatory Projectblackshock98% (47)
- Resume Civil EngineerDocument3 pagesResume Civil EngineerHarpreet SinghNo ratings yet
- ME2121 Finals ReviewDocument4 pagesME2121 Finals ReviewSherman LiamNo ratings yet
- National Models of HRM: Europe and USADocument31 pagesNational Models of HRM: Europe and USAKwiz MichaelNo ratings yet
- WEBB - The Physics of Three-Dimensional Radiation TherapyDocument368 pagesWEBB - The Physics of Three-Dimensional Radiation TherapyMiguelNo ratings yet
- Isothermal Gas Expansion: P V T P V TDocument4 pagesIsothermal Gas Expansion: P V T P V Twolfofphysics08IPMP01No ratings yet
- Thermodynamic Lesson 2Document7 pagesThermodynamic Lesson 2kelebekkNo ratings yet
- Expansions, Energy, Enthalpy Isothermal Gas Expansion: P V T P V TDocument7 pagesExpansions, Energy, Enthalpy Isothermal Gas Expansion: P V T P V TNaveen NaviNo ratings yet
- Sasi Institute of Technology EnineeringDocument6 pagesSasi Institute of Technology EnineeringHamu NalaNo ratings yet
- ProcessDocument5 pagesProcessNarcisse Serge NouadjepNo ratings yet
- Solution#1 PDFDocument4 pagesSolution#1 PDFuchnnaNo ratings yet
- (Physics, Thermal) - Solutions To Statistical Mechanics ProblemsDocument7 pages(Physics, Thermal) - Solutions To Statistical Mechanics ProblemsabartoskiNo ratings yet
- First Law (Contd.) : P V WorkDocument5 pagesFirst Law (Contd.) : P V Workashish44571No ratings yet
- ThermodynamicsDocument9 pagesThermodynamicssamir boseNo ratings yet
- Example-Water Flow in A PipeDocument13 pagesExample-Water Flow in A PipecristinelbNo ratings yet
- Chapter 6Document11 pagesChapter 6Analie Buerano SagunNo ratings yet
- Thermodynamics Intro and The First LawDocument17 pagesThermodynamics Intro and The First Lawrin rinNo ratings yet
- Unit 2 First Law-Closed System ProblemsDocument11 pagesUnit 2 First Law-Closed System Problemspiravi66No ratings yet
- BIS 154 - Mech Eng. 2 - Lecture 3.ppsxDocument32 pagesBIS 154 - Mech Eng. 2 - Lecture 3.ppsxMohamed NadaNo ratings yet
- Coupling of Elasticity, Flow and Material BalanceDocument29 pagesCoupling of Elasticity, Flow and Material BalanceDicky AlviansyahNo ratings yet
- Report-Open-Hydraulics-Energy EquationDocument8 pagesReport-Open-Hydraulics-Energy EquationJohn Eric CabañaNo ratings yet
- 351 F 22 Exam EquationsDocument1 page351 F 22 Exam EquationsEdaNo ratings yet
- Chapter 2 BDocument11 pagesChapter 2 BStefanPerendijaNo ratings yet
- Thermodynamics Formulae BookletDocument2 pagesThermodynamics Formulae BookletwardeqNo ratings yet
- RTT Mass, EnergyDocument5 pagesRTT Mass, EnergyPatrick Joseph RoblesNo ratings yet
- Lecture 2 EDocument8 pagesLecture 2 EMihai MirceaNo ratings yet
- Q W U H S: Isotérmico DT 0 Isobárico DP 0 Isocórico DV 0 Adiabático DQ 0Document2 pagesQ W U H S: Isotérmico DT 0 Isobárico DP 0 Isocórico DV 0 Adiabático DQ 0Aldasaurio SPNo ratings yet
- Physical Work 3Document25 pagesPhysical Work 3ScribdTranslationsNo ratings yet
- 102MAE Thermodynamics Formula SheetDocument2 pages102MAE Thermodynamics Formula SheetBogdan ProfirNo ratings yet
- Thermodynamics FormulaDocument9 pagesThermodynamics FormulaJayvie TumangNo ratings yet
- Chapter 3 - Section B - Non-Numerical SolutionsDocument12 pagesChapter 3 - Section B - Non-Numerical SolutionsAwaludin R FirmanshahNo ratings yet
- Ayuda para Balance de Masa y MomentumDocument4 pagesAyuda para Balance de Masa y MomentumalvaroNo ratings yet
- HW5 SolDocument3 pagesHW5 SoloppipxNo ratings yet
- Physics - Solution Set For Homework Book Chapter - 15 KTG & ThermodynamicsDocument4 pagesPhysics - Solution Set For Homework Book Chapter - 15 KTG & ThermodynamicsKishorNo ratings yet
- Compressible Flow Through Nozzles and Diffusers: V DT V D V VDocument14 pagesCompressible Flow Through Nozzles and Diffusers: V DT V D V VCamilo SantacruzNo ratings yet
- Equations FinalExamDocument5 pagesEquations FinalExamel locassinNo ratings yet
- Thermodynamic Processes and DerivationDocument10 pagesThermodynamic Processes and DerivationAbenayaNo ratings yet
- Formulário - Mecânica Dos Fluidos (MEE210/MEE620/MEE630)Document2 pagesFormulário - Mecânica Dos Fluidos (MEE210/MEE620/MEE630)Victor TavaresNo ratings yet
- Αντστροφείς MosDocument37 pagesΑντστροφείς MosvinothrathinamNo ratings yet
- Formelsammlung - ZustandsänderungenDocument2 pagesFormelsammlung - ZustandsänderungenTrần Nguyên KhôiNo ratings yet
- Formula Sheet + Charts Must Be Returned A3er The ExamDocument19 pagesFormula Sheet + Charts Must Be Returned A3er The ExamRaphael ErfeNo ratings yet
- Termodinamica ch03Document35 pagesTermodinamica ch03Rebeca AlmeidaNo ratings yet
- 7B Final Equation SheetDocument3 pages7B Final Equation SheetFangZiWenNo ratings yet
- Physics 112 Formulae IIIDocument3 pagesPhysics 112 Formulae IIISaied RajehaNo ratings yet
- Lecture 12+13 - Transient Waves On Transmission LinesDocument11 pagesLecture 12+13 - Transient Waves On Transmission Linessamer saeedNo ratings yet
- Allowed Cheat SheetDocument2 pagesAllowed Cheat SheetNguyễn Tương QuỳnhNo ratings yet
- Transmission Line Different Types of Transmission Line Transmission Line Per Unit Length Parameters Telegrapher Equation Power FlowDocument15 pagesTransmission Line Different Types of Transmission Line Transmission Line Per Unit Length Parameters Telegrapher Equation Power FlowdiptodevilNo ratings yet
- Total Differential, Rates of Change and Small ChangesDocument6 pagesTotal Differential, Rates of Change and Small ChangesChainarong TaepanichNo ratings yet
- Fluid Mechanics I Solution 4 Question 1: Problem P2.139: A B B C D DDocument8 pagesFluid Mechanics I Solution 4 Question 1: Problem P2.139: A B B C D Dcartoon_nateNo ratings yet
- Problem 4.158Document2 pagesProblem 4.158문jmtNo ratings yet
- Can IdealDocument3 pagesCan IdealHERRERA GAVINO LORIAN GERMANNo ratings yet
- PETE 411 Well Drilling: Surge and Swab PressuresDocument40 pagesPETE 411 Well Drilling: Surge and Swab PressuresBendali MehdiNo ratings yet
- Ideal GasDocument1 pageIdeal GasMike Raphy T. VerdonNo ratings yet
- Quiz 1 - MA SolutionsDocument6 pagesQuiz 1 - MA SolutionsabullaNo ratings yet
- Solución Parcial Mecánica de FluidosDocument5 pagesSolución Parcial Mecánica de FluidosIñigoNo ratings yet
- 20 562ln08Document8 pages20 562ln08sammy wanakaiNo ratings yet
- Compressibility PDFDocument2 pagesCompressibility PDFJuan Daniel CabreraNo ratings yet
- Finite Volume Method For One DimensionalDocument26 pagesFinite Volume Method For One Dimensionalscience1990No ratings yet
- The Spectral Theory of Toeplitz Operators. (AM-99), Volume 99From EverandThe Spectral Theory of Toeplitz Operators. (AM-99), Volume 99No ratings yet
- PTC Report Final DraftDocument35 pagesPTC Report Final DraftSaimKhanNo ratings yet
- Sodium Borohydride Fuel Cell AVRCDocument25 pagesSodium Borohydride Fuel Cell AVRCksksirhotNo ratings yet
- 111 SyllabusDocument5 pages111 SyllabusAlex BernsteinNo ratings yet
- Coursework HRM 2274Document10 pagesCoursework HRM 2274Nisha KarmakarNo ratings yet
- Bahay KuboDocument4 pagesBahay KuboJerome Amor AclanNo ratings yet
- Shazz ProjectDocument3 pagesShazz ProjectShahin NoorNo ratings yet
- 18.1 Areas: Definition, Names, and NotationsDocument2 pages18.1 Areas: Definition, Names, and NotationsRaghuSrinivasanNo ratings yet
- Panolin HLP Synth MSDSDocument4 pagesPanolin HLP Synth MSDSZarif All RightNo ratings yet
- Chapter 11 ScopingreviewsDocument2 pagesChapter 11 Scopingreviewsrodrigo weyllNo ratings yet
- 2C X 95CU POWER - Revised Datasheet Solar CableDocument1 page2C X 95CU POWER - Revised Datasheet Solar CableAashish MoyalNo ratings yet
- Rajratan Global WireDocument108 pagesRajratan Global WireReTHINK INDIANo ratings yet
- Batch ViiDocument2 pagesBatch ViiAmit KumarNo ratings yet
- Free Online Mock Test For MHT-CET BIOLOGY PDFDocument28 pagesFree Online Mock Test For MHT-CET BIOLOGY PDFBiologyForMHTCET75% (8)
- TLP - COMM1340 Business CommunicationsDocument12 pagesTLP - COMM1340 Business CommunicationsJäskãráñ ÇhåhàlNo ratings yet
- NLC Anecdotal Record Entry FinalDocument10 pagesNLC Anecdotal Record Entry FinalEthel Chrisia CejanoNo ratings yet
- Solving Linear Equations - FormulasDocument7 pagesSolving Linear Equations - FormulasMalhea VegeniaNo ratings yet
- DOE Et Al v. AUSTIN Et Al, Case Number 3 - 21-Cv-01211, 12 NOV 21, US District Court For The Northern District of Florida, Pages 12-15.cleanedDocument35 pagesDOE Et Al v. AUSTIN Et Al, Case Number 3 - 21-Cv-01211, 12 NOV 21, US District Court For The Northern District of Florida, Pages 12-15.cleanedAl Statilius100% (1)
- Letter of Recommendation Jumbo Sample Rev2Document2 pagesLetter of Recommendation Jumbo Sample Rev2Mohammed O. Al-Dushy100% (1)
- Write A Program For Error Detecting Code Using CRC-CCITT (16bit)Document39 pagesWrite A Program For Error Detecting Code Using CRC-CCITT (16bit)Megan_1234No ratings yet
- Item Anal Ni Pedro para Q3Document30 pagesItem Anal Ni Pedro para Q3norieesabayanNo ratings yet
- RCD 2 (Neu)Document9 pagesRCD 2 (Neu)Paul RoqueNo ratings yet