Professional Documents
Culture Documents
PSet 4 FL13
PSet 4 FL13
Uploaded by
cacer009Original Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
PSet 4 FL13
PSet 4 FL13
Uploaded by
cacer009Copyright:
Available Formats
MatS 2001 - Fall 2013 Problem Set 4:
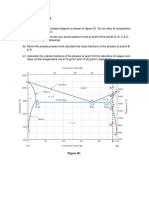
4-1 4-2 4-3 4-4 Callister 3.3 Callister 3.6 Callister 12.5 Calculate the packing fraction for sodium chloride based on the structure and ionic radii given in Ch 12. (a) Gold is FCC and has a lattice parameter of 0.40788 nm. Calculate the atomic radius of gold, in nanometers. (b) Callister 3.7; (c) Callister 3.9 (dont hand in but we will post the solution) 4-6. 4-7 4-8 4-9 Calculate the density of CaF2 (g/cm3)? Hint: You must establish the unit cell dimension. 1040 steel denotes iron that contains 0.40% by weight carbon. What percentage of atoms in 1040 steel are carbon? How many moles of M&Ms (peanut) would fit into the volume of the earth? (fun problem but dont need to hand in) (a) Callister 3.22; (b) Callister 3.23 4-10 Callister 3.30 parts (a), (b), (d) and (e) only 4-11 Callister 3.31 4-12 Callister 3.40 (a) - (d) 4-13 Callister 3.41
Due 10/11/13
4-5
FL12
Page 1 of 1
11/25/13 05:18:00 PM
You might also like
- Chemical Metallurgy Student PapersDocument3 pagesChemical Metallurgy Student PapersParesh SaksenaNo ratings yet
- Metallurgists-Quiz No.6 (: Mutiple Choice QuestionsDocument6 pagesMetallurgists-Quiz No.6 (: Mutiple Choice QuestionsRathnakrajaNo ratings yet
- Solid State Made BY KeshavPandey EngineerDocument6 pagesSolid State Made BY KeshavPandey EngineerVibhansh BhatiaNo ratings yet
- CCB 231 Supp ExamDocument6 pagesCCB 231 Supp ExamTumisang100% (1)
- 04 Askeland ChapDocument6 pages04 Askeland ChapEstudiante2346No ratings yet
- MT 1 Study QuestsDocument3 pagesMT 1 Study QuestsCaner AkkuşNo ratings yet
- CE3050 Tutorial01 2023Document2 pagesCE3050 Tutorial01 2023rockbottom 123No ratings yet
- Unit 2 - Chemistry - Final Review Q AnswersDocument6 pagesUnit 2 - Chemistry - Final Review Q Answersapi-269764684No ratings yet
- Chem Academy: Exercise - IDocument11 pagesChem Academy: Exercise - IHamit RanaNo ratings yet
- Seminario 1Document5 pagesSeminario 1Javier FrancoNo ratings yet
- MetalsDocument24 pagesMetals4D-31 WONG YUEN TSZNo ratings yet
- Chepter Wise QuestionsDocument279 pagesChepter Wise QuestionsAshok PradhanNo ratings yet
- Tutorial 4 MTSH 500 MemoDocument5 pagesTutorial 4 MTSH 500 MemoTEBATSONo ratings yet
- Class 9 Science 3Document7 pagesClass 9 Science 3chandralok_kumarNo ratings yet
- Multiple Choice QuestionsDocument7 pagesMultiple Choice QuestionsArya AnupamNo ratings yet
- Solid State & Surface Chemistry & Colloids - FDocument4 pagesSolid State & Surface Chemistry & Colloids - FAshwin BalajiNo ratings yet
- HW2 F06 KeyDocument6 pagesHW2 F06 KeyTorong VNo ratings yet
- Coordination Compounds 2014-22Document11 pagesCoordination Compounds 2014-22chithushree84No ratings yet
- ChemistryDocument8 pagesChemistryOMAR SHAHZAD KHANNo ratings yet
- Recitation 2 QuestionsDocument14 pagesRecitation 2 QuestionsfzfwsbyxrhNo ratings yet
- Chepter Wise QuestionsDocument513 pagesChepter Wise QuestionsAshok PradhanNo ratings yet
- Assignment 1 - Semester 2, 2017-18Document2 pagesAssignment 1 - Semester 2, 2017-18Student ServicesNo ratings yet
- DPP-13 (Coordination Compound) PDFDocument3 pagesDPP-13 (Coordination Compound) PDFAvishek BiswasNo ratings yet
- 2-Crystal Structure Practice Problems SolutionsDocument5 pages2-Crystal Structure Practice Problems Solutionsandrescasas850No ratings yet
- Chepter Wise QuestionsDocument240 pagesChepter Wise QuestionsVinay Tyagi100% (1)
- DPP 4 (Solid State) : Ans: (I) 2RDocument1 pageDPP 4 (Solid State) : Ans: (I) 2RajaxNo ratings yet
- Tutorial Questions - Physics Component - 11Document5 pagesTutorial Questions - Physics Component - 11CalvinhaoweiNo ratings yet
- JR IitDocument3 pagesJR IitGowri ShankarNo ratings yet
- BITSAT Chemistry SET 1Document8 pagesBITSAT Chemistry SET 1Sara MannNo ratings yet
- Sample Metals QuestionsDocument3 pagesSample Metals Questionsmn23sroaNo ratings yet
- Pearson Edexcel International IGCSE Examination: November 2021 ChemistryDocument6 pagesPearson Edexcel International IGCSE Examination: November 2021 ChemistryKim KatNo ratings yet
- ChemistryDocument128 pagesChemistryharshit jakharNo ratings yet
- Engineering MaterialsDocument47 pagesEngineering Materialsvikas2504100% (1)
- Memorandum Assignment 3Document2 pagesMemorandum Assignment 3Michael G ErasmusNo ratings yet
- 1 Crystal Defects - Ch04Document72 pages1 Crystal Defects - Ch04Kimberly Joy FerrerNo ratings yet
- Document From Vipin SinghDocument5 pagesDocument From Vipin SinghShashwatNo ratings yet
- Solid State-1Document12 pagesSolid State-1Ayush KumarNo ratings yet
- HT TP: //qpa Pe R.W But .Ac .In: 2013 Materials Science and TechnologyDocument7 pagesHT TP: //qpa Pe R.W But .Ac .In: 2013 Materials Science and TechnologyPuspendu Roy ChowdhuryNo ratings yet
- XII Chemistry QuestionBank Hathim HssliveDocument9 pagesXII Chemistry QuestionBank Hathim HsslivekeerthyNo ratings yet
- Neet Model - 6Document20 pagesNeet Model - 6krishna kamleshNo ratings yet
- Solid State Physics MCQsDocument7 pagesSolid State Physics MCQsAhsan MoinNo ratings yet
- Quiz 1 MSDocument11 pagesQuiz 1 MSApurva RakeshNo ratings yet
- Cordite Factory Higher Secondary School, Aruvankadu Exam Xii STDDocument3 pagesCordite Factory Higher Secondary School, Aruvankadu Exam Xii STDASWIN SNo ratings yet
- Assignment Material ScienceDocument9 pagesAssignment Material ScienceSabir AliNo ratings yet
- 12 Chemistry Important Questions Solid State 01Document7 pages12 Chemistry Important Questions Solid State 01Shahariya ShejeerNo ratings yet
- SS 1Document7 pagesSS 1xanshahNo ratings yet
- The Solid State: Unit-1Document7 pagesThe Solid State: Unit-1Rams ChanderNo ratings yet
- Solid State Revision SheetDocument6 pagesSolid State Revision SheetRumaysa -No ratings yet
- Chapter 9, Problem 1Document7 pagesChapter 9, Problem 1Cam MillerNo ratings yet
- GATE Metallurgical Engineering 2007Document15 pagesGATE Metallurgical Engineering 2007RATHIRAM NAIKNo ratings yet
- Catalyst: For Iit-Jee/ Aieee/ Neet/ Kvpy/ OlympiadDocument11 pagesCatalyst: For Iit-Jee/ Aieee/ Neet/ Kvpy/ OlympiadSerious BlackNo ratings yet
- Defect ProblemsDocument8 pagesDefect Problemsndreddy_pu100% (2)
- Instruction: Answer Number 1 and Any 2 Questions.: TheoryDocument2 pagesInstruction: Answer Number 1 and Any 2 Questions.: TheoryPrince CarrintonNo ratings yet
- Solid StateDocument2 pagesSolid StateKamal KishoreNo ratings yet
- Tutorial 1 (Solidification IMSE) QuestionsDocument1 pageTutorial 1 (Solidification IMSE) QuestionsNor MarzidaNo ratings yet
- Solid StateDocument16 pagesSolid StatememepepedankNo ratings yet
- Solid StateDocument16 pagesSolid StatePrahasNo ratings yet
- Unusual Structures and Physical Properties in Organometallic ChemistryFrom EverandUnusual Structures and Physical Properties in Organometallic ChemistryNo ratings yet
- Novel Carbon Materials and Composites: Synthesis, Properties and ApplicationsFrom EverandNovel Carbon Materials and Composites: Synthesis, Properties and ApplicationsXin JiangNo ratings yet