Professional Documents
Culture Documents
Calculating Two-Phase Pressure Drop
Uploaded by
rondonjjOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Calculating Two-Phase Pressure Drop
Uploaded by
rondonjjCopyright:
Available Formats
Chemical Processing, 2000 Fluid Flow Annual
Calculating Two-Phase Pressure Drop
Scott S. Haraburda, PE (scott.haraburda@gepex.ge.com) Production Engineer, GE Plastics, Mt. Vernon, IN And Steve Chafin (schafin@rivercityeng.com) Consulting Process Engineer, River City Engineering, Lawrence, KS Fluid flow concerns are quite prevalent among systems that handle chemicals, either in the liquid or the vapor state. An important parameter for characterizing the energy of the fluid flowing within a contained system, such as pipes, is pressure. This pressure becomes important for designing pipe sizes, determining pump requirements, and addressing safety concerns. Often the fluid is flowing as both liquid and gas.
Flow Type
The flow type of two-phase liquid-gas flow can be characterized into one of seven types shown in Figure 1. These types could be predicted using the following process parameters: Gas density ratio Liquid density ratio Viscosity ratio (gas ratio) = (gas) / (air) (liquid ratio) = (liquid) / (water) (ratio) = (liquid) / (water)
Surface tension ratio (ratio) = (liquid) / (water) Mass Flux MF (liquid or gas) = F(liquid or gas) / (3600*AR)
To determine which type of flow exists, use the following coefficients in Figure 1, which is a flow-pattern plot. Y (lb/sec ft2) = MF(gas) / [ (gas ratio) * (liquid ratio)] 0.5 X = MF(liquid) * [ (gas ratio) * (liquid ratio)] 0.5 * (ratio) / [MF(gas) * (ratio) 3 * (liquid ratio) 2]
5-Step Pressure Drop Calculation
The following steps can be used. Three examples will be provided, two oil - hydrogen mixtures and one ethanol - air mixture. The following steps have been replicated in the downloadable MS Excel spreadsheet at http://www.rivercityeng.com.
http://www.chemicalprocessing.com
Page 1 of 8
Chemical Processing, 2000 Fluid Flow Annual Step 1: Select the pipe parameters. When choosing the nominal pipe size, ensure that you have the appropriate inside diameter, based upon the pipe schedule. For the provided examples, a 4 inch standard pipe will be used for the oil - hydrogen mixtures and a 1 inch standard pipe will be used for the ethanol - air mixture. Both have an absolute roughness () of 0.0018 inches. Pipe Area AR = ( * d2) / 576
Step 2: Obtain the process parameters. The important properties are flow rate (F), safety factor (SF), density (), viscosity (), and surface tension (). The first example has a 5,000 lb/hr flow, a 51.85 lb/ft3 density, a 15 cP viscosity, and a 20 dynes / cm surface tension for the liquid (oil). The gas (hydrogen) is flowing at 800 lb/hr, with a 0.1420 lb/ft3 density and a 0.012 cP viscosity. The second example is the same as the first, with the exception that the liquid flow is 140,000 lb/hr. The third example has a 158.8 lb/hr flow, a 61.3 lb/ft3 density, a 1.07 cP viscosity, and a 51.4 dynes/cm surface tension for the liquid (ethanol). The gas (air) is flowing at 198.4 lb/hr, with a 0.0749 lb/ft3 density and a 0.0181 cP viscosity. For all three examples, there is no safety factor (SF=1). Step 3: Calculate the single phase line sizing pressure drop. The following equations are used to calculate this pressure drop for both the liquid and the gas phase flow. Velocity Reynolds Number Friction Factor where Pressure Drop v = F * SF / (3600 * * AR) Re = 19.83 * F * SF / ( * d * ) f = 64 / Re for Re < 2100 f = 8 * [(8/Re) 12 + 1/(A + B)1.5]1/12 A = [2.457 * ln(1 / ((7/Re)0.9 + 0.27 * / d))]16 B = (37,530/Re)16 P = 4.167 * f * v2 * / (gc * d) (this is in per 100 ft)
The first example has a 0.3 ft/sec velocity, a 523 Reynolds Number, a friction factor of 0.122 and a pressure drop of 0.02 psi / 100 ft of pipe for the liquid. The gas is flowing at 17.7 ft/sec, with a Reynolds Number of 105,000, a friction factor of 0.020 and a pressure drop of 0.03 psi / 100 ft. The second example has the same gas properties as the first example. However, the liquid is flowing at 8.48 ft/sec, with a Reynolds Number of 14,600, a friction factor of 0.029 and a pressure drop of 3.47 psi / 100 ft. The third example has a 0.12 ft/sec velocity, a 893 Reynolds Number, a friction factor of 0.072 and a pressure drop of 0.01 psi / 100 ft. The gas is flowing at 122.6 ft /sec, with a Reynolds Number of 66,000, a friction factor of 0.025 and a pressure drop of 3.53 psi / 100 ft.
http://www.chemicalprocessing.com
Page 2 of 8
Chemical Processing, 2000 Fluid Flow Annual Step 4: Calculate the two phase line sizing properties. The density, velocity, and viscosity are averaged for a characteristic property of the combined phases in the fluid flow. And, the resulting two-phase Reynolds Number is calculated. The following equations are used: Avg. density (average) = (F(gas) + F(liquid))/(F(gas)/ (gas) + F(liquid)/ (liquid)) Avg. velocity v(average) = (F(gas) + F(liquid)) / ( (average) * AR) Avg. viscosity (average) = (F(gas) + F(liquid))/(F(gas)/ (gas) + F(liquid)/ (liquid)) Figure 2 depicts values for this step and the next step for the examples provided. Step 5: There are three different types of two-phase pressure drop correlations. These are determined by the viscosity ratio and the mass flux. a. For viscosity ratios greater than 1000 and a mass flux greater than 20.5, use the Chisholm-Baroczy (C-B) method [see example 1]. The C-B method is unique in that the pressure drops for each of the phases are calculated assuming that the total mixture flows as either liquid or gas. Therefore: F(total) = F(liquid) +F(gas) MF = F(total) / (3600*AR) It should be noted that the Reynolds number and friction factor for each phase is also calculated assuming it is a function of total mass. Re(liquid or gas) = [F(total), SF, d, (liquid or gas)] f (liquid or gas) = [Re(liquid or gas), / d] P(liquid or gas) = 4.167 * f * MF2 / (gc * (liquid or gas) * d) (this is in per 100 ft) A pressure ratio is calculated: PR = [ P(gas) / P (liquid)]0.5 Using this pressure ratio, a C-B constant is calculated: CB = 24.9 / MF 0.5 CB = 235.3 / (PR * MF 0.5) CB = 6788.5 / (PR2 * MF 0.5) for PR < 9.5 for 9.5 < PR < 28 for PR > 28
http://www.chemicalprocessing.com
Page 3 of 8
Chemical Processing, 2000 Fluid Flow Annual Now, the C-B pressure correction factor and the associated two-phase pressure drop is calculated: (C-B) = 1 + (PR2 - 1) * (CB * (xg((2-n)/2) ) * ((1-xg) ((2-n)/2) ) + xg (2-n) ) Where xg = F(gas) / (F(gas) + F(liquid)) and n=0.25 P (C-B) = 4.167 * (C-B) * f(liquid) * MF2 / (gc * (liquid) * d) (this is in per 100 ft) b. For viscosity ratios greater than 1000 and a mass flux less than 20.5, use the Lockhart - Martinelli (L-M) method [see example 2]. The Reynolds Number for both the liquid and the gas are used. Unlike the C-B method, the separate pressure drops for both the liquid and the gas are used explicitly, along with the pressure ratio. Using these, a unique L-M pressure correction factor for each phase is calculated. This requires the use of a different pressure factor than the C-B method: PR = ln [( P (liquid) / P (gas)) 0.5] b1. For Re(liquid) > 2100 and Re(gas) > 2100: (liquid) = 1.44-0.508*PR+0.0579*PR2-0.000376*PR3-0.000444*PR4 (gas) = 1.44+0.492*PR+0.0577*PR2-0.000352*PR3-0.000432*PR4 b2. For Re(liquid) > 2100 and Re(gas) < 2100: (liquid) = 1.25-0.458*PR+0.067*PR2-0.00213*PR3-0.000585*PR4 (gas) = 1.25+0.542*PR+0.067*PR2-0.00212*PR3-0.000583*PR4 b3. For Re(liquid) < 2100 and Re(gas) > 2100: (liquid) = 1.24-0.484*PR+0.072*PR2-0.00127*PR3-0.00071*PR4 (gas) = 1.24+0.516*PR+0.072*PR2-0.00126*PR3-0.000706*PR4 b4. For Re(liquid) < 2100 and Re(gas) < 2100: (liquid) = 0.979-0.444*PR+0.096*PR2-0.00245*PR3-0.00144*PR4 (gas) = 0.979+0.555*PR+0.096*PR2-0.00244*PR3-0.00144*PR4 Now, a separate pressure drop is calculated for each phase: P(liquid1) = [exp[ (liquid)]]2 * P (liquid) P(gas1) = [exp[ (gas)]]2 * P (gas) Then, the estimated two-phase pressure drop is the maximum of these: P(L-M) = max { P(liquid1), P (gas1)}
http://www.chemicalprocessing.com
Page 4 of 8
Chemical Processing, 2000 Fluid Flow Annual c. Finally, for viscosity ratios less than 1000, use the Friedel method [see example 3]. In addition to the Reynolds number, the Froude number and Weber numbers are used. In the following equations, be sure to use the mass flux of the total mass (liquid+gas) flowing in the pipe. Froude Number Weber Number Fr = 12 * MF2 / (gc * (average)2 * d) We = 37.8 * d * MF2 / ( (average) * )
A gas mass ratio and two Friedel coefficients are also used: Gas mass ratio xg = F(gas) / [F(liquid) + F(gas)] Calculate 1 for both phases using the Reynolds number calculated for each phase: Friedel coefficient 1 1 = 64 / Re 1 = [0.86859 * ln(Re/(1.964*ln(Re) - 3.8215))] -2 for Re < 1055 for Re > 1055
Friedel coefficient 2 2 = (1- xg)2 + (xg 2) * [ (liquid) * 1(gas) / { (gas) * 1(liquid)}] Two pressure correction factors are used, one for horizontal flow (which includes vertical up) and another for vertical down flow. The correlation for horizontal flow is: (F) = 2+3.24* xg 0.78 *(1- xg) 0.24 *( (liquid)/ (gas)) 0.91 * ( (gas)/ (liquid)) 0.19 *(1- (gas)/ (liquid)) 0.70 *Fr -0.045 *We -0.035 The correlation for vertical down flow is: (F) = 2+38.5* xg 0.75 *(1- xg) 0.314 *( (liquid)/ (gas)) 0.86 * ( (gas)/ (liquid)) 0.73 *(1- (gas)/ (liquid)) 6.84 *Fr -0.0001 *We -0.037 Finally, the two-phase pressure drop is then calculated using the following: P(F) = 4.167 * (F) * 1(liquid) * MF2 / (gc * (liquid) * d) (this is in per 100 ft)
http://www.chemicalprocessing.com
Page 5 of 8
Chemical Processing, 2000 Fluid Flow Annual
Symbol List
Symbol
A AR B CB d f Fr gc F MF n P PR Re SF v We xl xg X Y
Definition
Friction Factor number Pipe Area Friction Factor number Chisholm-Baroczy constant Pipe Diameter Friction Factor Froude number Dimensional constant Mass flow Mass flux Chisholm-Baroczy constant Pressure Pressure ratio Reynolds number Safety factor (none = 1) Velocity Weber number Liquid Mass Ratio Gas Mass Ratio Dimensionless Flow Pattern Region coefficient Flow Pattern Region coefficient Surface Roughness Friedel coefficient Pressure Correction factor Density Surface Tension Viscosity
Units
none ft2 none dimensionless inch none none 32.174 lb ft / lbf sec2 lb / hour lb/ft2-sec Dimensionless Psi dimensionless none dimensionless feet / second none none none none pounds / sec ft2 inches none none lb / ft3 dynes / centimeter Centipoise
http://www.chemicalprocessing.com
Page 6 of 8
Chemical Processing, 2000 Fluid Flow Annual Figure 1: Flow Types
Type
Bubble (froth)
Description
Liquid with dispersed bubbles of gas.
Sketch
Plug
alternating plugs of gas and liquid in upper section of pipe.
Stratified
Liquid on the lower and gas on the upper section pipe separated by a smooth interface.
Wave
Same as stratified, except separated by a wavy interface traveling in the same direction of flow.
Slug
Annular
Similar to stratified, except the gas periodically picks up a wave and forms a bubbly plug. This flow can cause severe and dangerous vibrations because of the impact of the high-velocity slugs against the equipment. Gas in the center and liquid on the outer portion of the pipe.
Spray (dispersed)
Liquid droplets in the gas.
http://www.chemicalprocessing.com
Page 7 of 8
Chemical Processing, 2000 Fluid Flow Annual Figure 2: Example Results Example 1 2 1.012 16.895 18.01 26.19 0.087 1.853 105,000 119,000 1250 1250 18 442 L-M C-B 0.28 9.64
Property Average Density Average Velocity Average Viscosity Reynolds Number Viscosity Ratio Mass Flux Correlation Type Pressure Drop Horizontal Vertical down References: 1. 2. 3. 4. 5. 6. 7. 8.
3 0.135 122.72 0.032 66,900 59 17 Friedel 9.86 11.10
Baker, O., Oil & Gas J., Vol. 53, No. (12), 1954, pp. 185-190, 192, 195. Lockhart, R.W. and R.C. Martinelli, Chemical Engineering Progress, 1949, pp. 39-45. Chisholm D., Int. J. Heat Mass Transfer, Vol. 16, pp. 347-358. Friedel, L., "Improved Friction Pressure Drop Correlations for Horizontal and Vertical Two Phase Pipe Flow," European Two Phase Flow Group Meeting, Ispra, Italy, paper E2, 1979. Hewitt, G.F., Liquid-Gas Systems, Handbook of Multiphase Systems, Chapter 2, 1982. Walas, S.M., Chemical Process Equipment, Selection and Design, Butterworths, Massachusetts, 1988. Bennett, C., and J. Meyers, Momentum, Heat and Mass Transfer, 3rd Edition, McGraw-Hill, New York, 1982. Perry, R. and D. Green, Perry's Chemical Engineers' Handbook, 6th Edition, McGraw-Hill, New York, 1984.
http://www.chemicalprocessing.com
Page 8 of 8
You might also like
- Aga-3 Orifice (Api Chapter 14.3.1) - Iphone App - DocumentationDocument4 pagesAga-3 Orifice (Api Chapter 14.3.1) - Iphone App - DocumentationLuis MclaffertyNo ratings yet
- Pipe Flow Calculations PDFDocument12 pagesPipe Flow Calculations PDFharrypop418No ratings yet
- 4.47 The Expansion Factor, Y: P, AP P, P2Document18 pages4.47 The Expansion Factor, Y: P, AP P, P2Enrico GambiniNo ratings yet
- 230 Sp18 PREP5 Blank PDFDocument24 pages230 Sp18 PREP5 Blank PDFKhang HuynhNo ratings yet
- Isometric Gradient Social Media Strategy by SlidesgoDocument46 pagesIsometric Gradient Social Media Strategy by SlidesgoEwerton MazoniNo ratings yet
- PBDocument6 pagesPBdalton2003100% (1)
- Calculating Two-Phase Pressure Drop (Editado)Document8 pagesCalculating Two-Phase Pressure Drop (Editado)anon_37067086No ratings yet
- Validation Report On The 2-Phase Line SizingDocument18 pagesValidation Report On The 2-Phase Line SizingEbby Onyekwe100% (1)
- Advanced Flow AssuranceDocument123 pagesAdvanced Flow AssuranceThành Bk100% (5)
- AGA-3 Orifice - DocDocument4 pagesAGA-3 Orifice - Docsuci wulandariNo ratings yet
- Pressure Drop Evaluation Along PipelinesDocument23 pagesPressure Drop Evaluation Along PipelinespeweajeNo ratings yet
- RMS Inlet Pressure Calculation TheoryDocument22 pagesRMS Inlet Pressure Calculation TheoryRajesh SarkarNo ratings yet
- KOHASA ENGINEERING LINE SIZING CALCULATIONSDocument9 pagesKOHASA ENGINEERING LINE SIZING CALCULATIONSEkundayo JohnNo ratings yet
- Source Terms For Accidental Gas And Liquid Release RatesDocument9 pagesSource Terms For Accidental Gas And Liquid Release Ratesdei_sandeep7994No ratings yet
- Drilling Hydraulics ADocument63 pagesDrilling Hydraulics Asryn89100% (3)
- Pipe FlowDocument9 pagesPipe FlowStephen Mirdo83% (6)
- Gas LooplinestptDocument64 pagesGas Looplinestptniyo7No ratings yet
- Performance Evaluation of Air PreheaterDocument8 pagesPerformance Evaluation of Air PreheaterAndria MatthewsNo ratings yet
- Differential PR and Flow RelationshipDocument7 pagesDifferential PR and Flow RelationshipMY NAME IS NEERAJ..:):)No ratings yet
- Offshore Pipeline Hydraulic and Mechanical AnalysesDocument25 pagesOffshore Pipeline Hydraulic and Mechanical AnalysesEslam RedaNo ratings yet
- Chemical Engineering Laboratory IDocument54 pagesChemical Engineering Laboratory IAndini DamayantiNo ratings yet
- Line SizingDocument20 pagesLine SizingAhmed HassanNo ratings yet
- Flow in Circular Pipes: ObjectiveDocument35 pagesFlow in Circular Pipes: ObjectivemamunruetNo ratings yet
- Velocity and Pressure Drop in PipesDocument5 pagesVelocity and Pressure Drop in PipesManojkumar ThilagamNo ratings yet
- Petrowiki Pressure Drop EquationsDocument14 pagesPetrowiki Pressure Drop Equationsrasnowmah2012No ratings yet
- The Reynolds Number - Units in A Dimensionless Number: Pdhonline Course M476 (3 PDH)Document27 pagesThe Reynolds Number - Units in A Dimensionless Number: Pdhonline Course M476 (3 PDH)Neil Erwin A. RoselloNo ratings yet
- Multiphase Flow in Pipes, 2006, Critical Velocity, PresentacionDocument62 pagesMultiphase Flow in Pipes, 2006, Critical Velocity, PresentacionjoreliNo ratings yet
- Velocity and Pressure Drop in PipesDocument5 pagesVelocity and Pressure Drop in Pipeskiran9285No ratings yet
- Bottomholemodaloil PCDocument6 pagesBottomholemodaloil PCKimiko ShopNo ratings yet
- Liquid Holdup, MODELS PDFDocument14 pagesLiquid Holdup, MODELS PDFjoreliNo ratings yet
- Hydrodynamic Bearing Design ParametersDocument24 pagesHydrodynamic Bearing Design ParametersroquirogaNo ratings yet
- PHPA As A Frictional Pressure Loss Reducer and Its Pressure Loss EstimationDocument6 pagesPHPA As A Frictional Pressure Loss Reducer and Its Pressure Loss Estimationtariq82aliNo ratings yet
- Lockhart&Martinelli ImprovedDocument12 pagesLockhart&Martinelli Improvedrui_filho_12No ratings yet
- Compressible FlowDocument35 pagesCompressible FlowAngelica KartikaNo ratings yet
- Air Stripping Design ReportDocument17 pagesAir Stripping Design ReportShawn MauldinNo ratings yet
- Pipe Flow & Hydraulics Slide RuleDocument3 pagesPipe Flow & Hydraulics Slide RuleOtis ArmsNo ratings yet
- Piping Design and Operations Guideobook - Volume 1 PDFDocument86 pagesPiping Design and Operations Guideobook - Volume 1 PDFgamron89% (35)
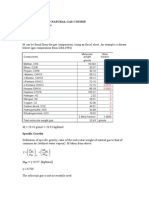
- Calculations in Natural Gas CourseDocument6 pagesCalculations in Natural Gas CourseHilmi ScpgzNo ratings yet
- Theory Pressure Drop Evaluation Along PipelinesDocument9 pagesTheory Pressure Drop Evaluation Along PipelinesNovaCastilloNo ratings yet
- CALIB053 - Mathcad 15 EquationsDocument8 pagesCALIB053 - Mathcad 15 Equationsilie_vlassaNo ratings yet
- Section 17 - Fluid Flow and PipingDocument11 pagesSection 17 - Fluid Flow and PipingCHANADASNo ratings yet
- Validation Report On The 2 Phase Line Sizing 3 PDFDocument18 pagesValidation Report On The 2 Phase Line Sizing 3 PDFJoseph MedinaNo ratings yet
- Man-Arian Flow Cad SoftwareDocument27 pagesMan-Arian Flow Cad Softwaresyahmi1337No ratings yet
- TPG 4140 CALCULATIONSDocument6 pagesTPG 4140 CALCULATIONSpatrickandreas77No ratings yet
- AGA8 DetailDocument3 pagesAGA8 DetailIndra RosandiNo ratings yet
- Well Completion and Stimulation - Chapter 3 Well Performance Analysis-NewDocument56 pagesWell Completion and Stimulation - Chapter 3 Well Performance Analysis-NewsouthliNo ratings yet
- Calibration Procedure KrissDocument12 pagesCalibration Procedure KrissALP69No ratings yet
- Choke Sizing & Propiedaes de Los FluidosDocument149 pagesChoke Sizing & Propiedaes de Los FluidosJose RojasNo ratings yet
- Pressure Drop CalculationDocument41 pagesPressure Drop CalculationFrancisco Rivas50% (2)
- 8.pressure Drop in Piping PDFDocument41 pages8.pressure Drop in Piping PDFStalin Apolo100% (1)
- Steady-State Flow of Gas Through Pipes: Du+ DV G G DZ +D (PV) +DQ D WDocument16 pagesSteady-State Flow of Gas Through Pipes: Du+ DV G G DZ +D (PV) +DQ D WAkshat TarateNo ratings yet
- Formulas and Calculations for Drilling, Production, and Workover: All the Formulas You Need to Solve Drilling and Production ProblemsFrom EverandFormulas and Calculations for Drilling, Production, and Workover: All the Formulas You Need to Solve Drilling and Production ProblemsNo ratings yet
- Southern Marine Engineering Desk Reference: Second Edition Volume IFrom EverandSouthern Marine Engineering Desk Reference: Second Edition Volume INo ratings yet
- Recommended Reference Materials for Realization of Physicochemical Properties: Pressure–Volume–Temperature RelationshipsFrom EverandRecommended Reference Materials for Realization of Physicochemical Properties: Pressure–Volume–Temperature RelationshipsE. F. G. HeringtonNo ratings yet
- Natural Gas Processing from Midstream to DownstreamFrom EverandNatural Gas Processing from Midstream to DownstreamNimir O. ElbashirNo ratings yet
- Equipo Mayor (Major Equipment List)Document4 pagesEquipo Mayor (Major Equipment List)rondonjjNo ratings yet
- January 2012 Revistas Técnica de Bombas PDFDocument76 pagesJanuary 2012 Revistas Técnica de Bombas PDFrondonjjNo ratings yet
- 937E - BS&B Systems PDFDocument4 pages937E - BS&B Systems PDFrondonjjNo ratings yet
- R. R. Evans Etal 2,780,361: Scum SkimmerDocument10 pagesR. R. Evans Etal 2,780,361: Scum SkimmerrondonjjNo ratings yet
- Cooling Tower Thermal DesignDocument106 pagesCooling Tower Thermal Designxuense100% (4)
- Standby 3 0 0 e K W 3 7 5 K V A 60 HZ 1800 RPM 240 Volts: Diesel Generator SetDocument6 pagesStandby 3 0 0 e K W 3 7 5 K V A 60 HZ 1800 RPM 240 Volts: Diesel Generator SetrondonjjNo ratings yet
- TP03ISA228Document12 pagesTP03ISA228rondonjjNo ratings yet
- INOFLAR PVDF Homopolymer MSDS EnglishDocument9 pagesINOFLAR PVDF Homopolymer MSDS EnglishDiadam SharmaNo ratings yet
- 414CC3 Excel Template Prelim Shell and Tube Heat Exchanger Design Si UnitsDocument3 pages414CC3 Excel Template Prelim Shell and Tube Heat Exchanger Design Si UnitsGuruh Mehra MulyanaNo ratings yet
- LNG Plant1Document32 pagesLNG Plant1Shadi ZuraikatNo ratings yet
- Open Channel Flow Gate Notes 65Document5 pagesOpen Channel Flow Gate Notes 65Saurabh SinghNo ratings yet
- Advances in Crystallization ProcessesDocument660 pagesAdvances in Crystallization ProcessesJosé Ramírez0% (1)
- STPM Trial 2009 Che Q&A KelantanDocument37 pagesSTPM Trial 2009 Che Q&A KelantanSimPorNo ratings yet
- Drag EquationDocument4 pagesDrag EquationMspamNo ratings yet
- Special Steels CB10FF: For Cold Deformation and BearingsDocument1 pageSpecial Steels CB10FF: For Cold Deformation and BearingsRollpass DesignNo ratings yet
- Actuator StepbystepDocument53 pagesActuator StepbystepQuang Huy VũNo ratings yet
- Chemistry For EngineersDocument5 pagesChemistry For EngineersErgie PaglinawanNo ratings yet
- Descriptive Physical Oceanography An IntroductionDocument3 pagesDescriptive Physical Oceanography An IntroductionAnonymous xdDh30QONo ratings yet
- Pondicherry University B.Sc. Physics Regulations & SyllabusDocument39 pagesPondicherry University B.Sc. Physics Regulations & SyllabusenochrajeshNo ratings yet
- Physics concepts and principlesDocument16 pagesPhysics concepts and principlesKalana GamageNo ratings yet
- Mineralogical and Geochemical Characteristics of Belevi Clay DepositsDocument13 pagesMineralogical and Geochemical Characteristics of Belevi Clay DepositsFOUTOUNo ratings yet
- Prism Pa Nitrogen Membrane SeparatorsDocument8 pagesPrism Pa Nitrogen Membrane SeparatorsjosalkNo ratings yet
- Experiment 3Document22 pagesExperiment 3Sophiah RachelleNo ratings yet
- Vetiver Distillation PDFDocument114 pagesVetiver Distillation PDFdummy dummyNo ratings yet
- Wacker Process Slides 2008Document5 pagesWacker Process Slides 2008Zakariya AdamNo ratings yet
- Astronomy 1619485741Document69 pagesAstronomy 1619485741Julio Arroyo GilNo ratings yet
- Basic Instrument SymbolsDocument7 pagesBasic Instrument Symbolssushant_jhawer100% (1)
- EVreporter October 2021 e MagazineDocument36 pagesEVreporter October 2021 e MagazinekarthikNo ratings yet
- CXC Chemistry Mixtures and Separation PowerpointDocument28 pagesCXC Chemistry Mixtures and Separation PowerpointJordanNo ratings yet
- Battery Thermal ManagementDocument20 pagesBattery Thermal ManagementvadanNo ratings yet
- 03.current Electricity FDocument53 pages03.current Electricity Fmahlom06No ratings yet
- PHD Thesis: STATIC FRICTION IN RUBBER-METAL CONTACTS WITH APPLICATION TO RUBBER PAD FORMING PROCESSESDocument183 pagesPHD Thesis: STATIC FRICTION IN RUBBER-METAL CONTACTS WITH APPLICATION TO RUBBER PAD FORMING PROCESSESJonathan Xie100% (1)
- Amema 2023Document4 pagesAmema 2023BRajesh ReddyNo ratings yet
- Condenser and feedwater performance testingDocument23 pagesCondenser and feedwater performance testingShambhu MehtaNo ratings yet