Professional Documents
Culture Documents
Atomic Physics 6
Uploaded by
Ramo ApuCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Atomic Physics 6
Uploaded by
Ramo ApuCopyright:
Available Formats
Page No.
6
23. A hydrogen like atom has one electron revolving round a stationary nucleus. The energy required to
excite the electron from 2
nd
to 3
rd
orbit is 47.2eV. The atomic number (Z) of the atom is
1) 3 2) 4 3) 5 4) 6
24. In hydrogen atom H
line arises due to transition n = 3
n = 2. In the spectrum of singly ionised
helium there is a line having the same wavelength as the H
line. This is due to the transition.
1) n =3
n =2 2) n =2
n =1 3) n =5
n =3 4) n =6
n =4
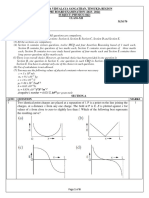
25. The figure shows the variation of photocurrent with anode potential for a photosensitive surface for
three different radiations. Let ,
a b
and
c
be the intensities and f
a
, f
b
and f
c
be the
frequencies for the curves A, B and C respectively
1) f
a
=f
b
and I
a
I
c
2) f
a
=f
c
and I
a
=I
c
I
C
B
A
V
3) f
a
=f
b
and I
a
=I
b
4) f
b
=f
c
and I
b
=I
c
26. Let K
1
be the maximum kinetic energy of photoelectrons emitted by light of wavelength
1
and K
2
corresponding to wavelength
2
. If
1 2
2 then
1) 2K
1
=K
2
2) K
1
=2K
2
3) K
1
<K
2
/2 4) K
1
>2K
2
You might also like
- MCAT Practice PsDocument4 pagesMCAT Practice PsStephen CampbellNo ratings yet
- Class-Xii - Final Cbse Board Physics Sample Paper-2Document7 pagesClass-Xii - Final Cbse Board Physics Sample Paper-2kamali.v777No ratings yet
- Prestige Public School: Periodic Test - III (2021-22)Document3 pagesPrestige Public School: Periodic Test - III (2021-22)Lucifer GamingNo ratings yet
- Aipmt 2005 PrelimsDocument36 pagesAipmt 2005 PrelimsKiran Raj RNo ratings yet
- General Instructions:: Sample Question Paper - 23 Physics (042) Class-XII, Session: 2021-22Document6 pagesGeneral Instructions:: Sample Question Paper - 23 Physics (042) Class-XII, Session: 2021-22abcdNo ratings yet
- Test61 QPDocument6 pagesTest61 QPnareshsuja123No ratings yet
- Model Test Paper Physics CBSE Class XII 2023 - I Part IIDocument4 pagesModel Test Paper Physics CBSE Class XII 2023 - I Part IIAnanthakrishnan Tinneveli VNo ratings yet
- Aipmt 2005 PrelimsfDocument36 pagesAipmt 2005 PrelimsfdineshhissarNo ratings yet
- SR Neet Star Super Chaina (Cbse) (Pt-1) Q.P Ex - Dt. 17.07.2023Document24 pagesSR Neet Star Super Chaina (Cbse) (Pt-1) Q.P Ex - Dt. 17.07.2023dhruvi.v91No ratings yet
- Physics SQP - 05 Latest (2023) Class 12 CBSEDocument27 pagesPhysics SQP - 05 Latest (2023) Class 12 CBSEmr mindNo ratings yet
- Sem VI - PHSH - CC13 PDFDocument3 pagesSem VI - PHSH - CC13 PDFÂřîjìť PāłNo ratings yet
- Topic Wise Review Cpp-I-As - PMDDocument4 pagesTopic Wise Review Cpp-I-As - PMDSaksham PanghalNo ratings yet
- Ques PaperDocument10 pagesQues Papergupta.sg003No ratings yet
- Sample Paper 2 Word FileDocument12 pagesSample Paper 2 Word FileAtul DubeyNo ratings yet
- Physics SQPDocument10 pagesPhysics SQPPankaj SinghNo ratings yet
- Phy Assi PDFDocument3 pagesPhy Assi PDFvijay ladeNo ratings yet
- Test Paper 9Document7 pagesTest Paper 9rajeshsharma4121No ratings yet
- QP12PHY02PB23Document8 pagesQP12PHY02PB23Ayush TomarNo ratings yet
- Test Paper 7Document10 pagesTest Paper 7ashishgambhir1986No ratings yet
- INPhO 2001Document6 pagesINPhO 2001gudapudi ramaniNo ratings yet
- Physics Pre-Board I 2022-23Document6 pagesPhysics Pre-Board I 2022-23Ayushi KayalNo ratings yet
- IAS Previous Year Test PapersDocument4 pagesIAS Previous Year Test PapersRavinder Singh100% (2)
- Pre BoardDocument15 pagesPre Boardfakesmilelover07No ratings yet
- Physics Pre BoardDocument10 pagesPhysics Pre Boardizumigamer69No ratings yet
- Aipmt 2009 Question PaperDocument38 pagesAipmt 2009 Question PaperPooja100% (1)
- XII Physics QPDocument8 pagesXII Physics QPsinghbharat50726No ratings yet
- CLASS XII PRE BOARD Physics QP 2023-24Document19 pagesCLASS XII PRE BOARD Physics QP 2023-24suprajabhupalanNo ratings yet
- LT-23 - SPL - GP1-MED-Home Work - Structure of Atom 29-07-21Document7 pagesLT-23 - SPL - GP1-MED-Home Work - Structure of Atom 29-07-21orisNo ratings yet
- 12CBSE-Physics-Model - QPDocument11 pages12CBSE-Physics-Model - QPGuestNo ratings yet
- Class XII Physics Support Material Final-1 - Removed - RemovedDocument70 pagesClass XII Physics Support Material Final-1 - Removed - RemovedIshu RaoNo ratings yet
- Physics Class Xii Sample Paper Test 12 For Board Exam 2024Document5 pagesPhysics Class Xii Sample Paper Test 12 For Board Exam 2024xkryxxzNo ratings yet
- Paper - 4Document24 pagesPaper - 4Sunil KumarNo ratings yet
- Xii-Board 3RD 33% Physics QP - 18.11.2023Document7 pagesXii-Board 3RD 33% Physics QP - 18.11.2023eashwarsiddhaNo ratings yet
- 2.physics Qp-XiiDocument6 pages2.physics Qp-XiiEMMANUEL PHILIP REJI CLASS XNo ratings yet
- Atomic Structure FDocument10 pagesAtomic Structure FRaju SinghNo ratings yet
- 12 Rev MCQ Ans KeyDocument9 pages12 Rev MCQ Ans Keyselva.nishanth2006No ratings yet
- AIIMS Full Paper 2007Document33 pagesAIIMS Full Paper 2007Sombir Ahlawat100% (1)
- Atomic Structure PDFDocument19 pagesAtomic Structure PDFggk2013100% (3)
- Ugc Net Physics1Document2 pagesUgc Net Physics1kvspayyanurNo ratings yet
- 01 - Physics - January 2006Document6 pages01 - Physics - January 2006Bernardo Gonzalez GarciaNo ratings yet
- Cmp1+Tut5 - in A Free Electron ModelDocument1 pageCmp1+Tut5 - in A Free Electron ModelAchintya DharNo ratings yet
- Problems 42Document12 pagesProblems 42Maurice KingNo ratings yet
- P Bxii Physics 2023-24Document9 pagesP Bxii Physics 2023-2410B16 Kanchan Waghulde.No ratings yet
- E6 AnswersDocument20 pagesE6 AnswersgovardhanNo ratings yet
- Phy Set-2 QPDocument5 pagesPhy Set-2 QPSiddhartha HadimaniNo ratings yet
- NEET Exam 2005 Original Question Paper and Answer Key Click HereDocument39 pagesNEET Exam 2005 Original Question Paper and Answer Key Click HereBalaji ElumalaiNo ratings yet
- AIIMS MBBS Entrance Examination 2004 Solved Question PaperDocument36 pagesAIIMS MBBS Entrance Examination 2004 Solved Question PaperAditya GoelNo ratings yet
- Physics Sample Papers 2022-23 QPDocument42 pagesPhysics Sample Papers 2022-23 QPOJASisLiveNo ratings yet
- Bhavan's Netaji Subhash Physics Pre Board 1Document9 pagesBhavan's Netaji Subhash Physics Pre Board 1niladriputatunda1No ratings yet
- Test Paper 8Document8 pagesTest Paper 8rajeshsharma4121No ratings yet
- Physics SQPDocument7 pagesPhysics SQPGopa DeyNo ratings yet
- 2020 12 SP PhysicsDocument22 pages2020 12 SP PhysicsRiya NinanNo ratings yet
- SAMPLE QUESTION PAPER - XII - Physics2023-24Document6 pagesSAMPLE QUESTION PAPER - XII - Physics2023-24Nandita SharmaNo ratings yet
- Assignment 1Document3 pagesAssignment 1MainzaNo ratings yet
- Nta Abhyas Neet Mock Test - 21: PhysicsDocument39 pagesNta Abhyas Neet Mock Test - 21: PhysicsLord SivaNo ratings yet
- 2755IIT JEE Physics Question Paper-1999Document12 pages2755IIT JEE Physics Question Paper-1999SARTHAK MISHRA X-E ROLL NO - 47No ratings yet
- OlympiadDocument2 pagesOlympiadRajeev GangwarNo ratings yet
- Problems 42Document12 pagesProblems 42mail2sgarg_841221144No ratings yet
- Electron Beam-Specimen Interactions and Simulation Methods in MicroscopyFrom EverandElectron Beam-Specimen Interactions and Simulation Methods in MicroscopyNo ratings yet
- Advances in Structure Research by Diffraction Methods: Fortschritte der Strukturforschung mit BeugungsmethodenFrom EverandAdvances in Structure Research by Diffraction Methods: Fortschritte der Strukturforschung mit BeugungsmethodenR. BrillNo ratings yet
- Atomic Physics CDocument1 pageAtomic Physics CRamo ApuNo ratings yet
- Atomic Physics 8Document1 pageAtomic Physics 8Ramo ApuNo ratings yet
- Atomic Physics 7Document1 pageAtomic Physics 7Ramo ApuNo ratings yet
- Atomic Physics ADocument1 pageAtomic Physics ARamo ApuNo ratings yet
- Atomic Physics 4Document1 pageAtomic Physics 4Ramo ApuNo ratings yet
- Phy 5Document1 pagePhy 5Ramo ApuNo ratings yet
- Phy 6Document1 pagePhy 6Ramo ApuNo ratings yet
- Physics Obj1Document1 pagePhysics Obj1Ramo ApuNo ratings yet
- Electric Charges and Fields: E KQR RDocument1 pageElectric Charges and Fields: E KQR RRamo ApuNo ratings yet
- Phy 8Document1 pagePhy 8Ramo ApuNo ratings yet
- Phy qp-5Document1 pagePhy qp-5Ramo ApuNo ratings yet
- Electromagnetics Wavelength: S EB E CB EDocument1 pageElectromagnetics Wavelength: S EB E CB ERamo ApuNo ratings yet
- Physical OpticsDocument2 pagesPhysical OpticsRamo ApuNo ratings yet
- In Vacuum Are in Phase Initially. Then The First Ray: 1) (1/5) Radian 2) 4 Radian 3) 5 Radian 4) 6 RadianDocument1 pageIn Vacuum Are in Phase Initially. Then The First Ray: 1) (1/5) Radian 2) 4 Radian 3) 5 Radian 4) 6 RadianRamo ApuNo ratings yet
- 1) 2I 2) 4I 3) 5I 4) 6I: Is K. What Will Be The Intensity at The Point Where Path Difference IsDocument1 page1) 2I 2) 4I 3) 5I 4) 6I: Is K. What Will Be The Intensity at The Point Where Path Difference IsRamo ApuNo ratings yet
- Phy qp-2Document1 pagePhy qp-2Ramo ApuNo ratings yet
- Gauss'S Law: N M C N M C ADocument1 pageGauss'S Law: N M C N M C ARamo ApuNo ratings yet