Professional Documents
Culture Documents
Picnokosong Picno + HCL HCL
Uploaded by
Anonymous a8990NEOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Picnokosong Picno + HCL HCL
Uploaded by
Anonymous a8990NECopyright:
Available Formats
P1
LEMBAR PERHITUNGAN
A. PERHITUNGAN REAGEN
Larutan NaOH 0,2 N sebanyak 10 Liter
Larutan HCl 0,1 N sebanyak 200 ml
mpicnokosong
: 22,7 gr
mpicno + HCl
: 51,2 gr
mHCl
: 28,5 gr
= 1,14 gr/ml
N=
0,1 N =
V HCl = 2,56 ml
B. PERHITUNGAN FRAKSI RUANG KOSONG
C. OPERASI ABSORBSI
Laju Alir NaOH 20 ml/menit
Z
= 6,5 cm = 0,065 m
= 5,8 bar
P1
Pkompresor
= 2,16 bar
= 3,3310-4 L/sec
Laju Alir NaOH 30 ml/menit
Z
= 1,5 cm = 0,015 m
= 5,8 bar
Pkompresor
= 2,16 bar
= 510-4 L/sec
Laju Alir NaOH 40 ml/menit
Z
= 1,5 cm = 0,015 m
= 5,8 bar
Pkompresor
= 2,16 bar
Q2
= 6,6710-4 L/sec
D. PERHITUNGAN LAJU ALIR
Massa jenis raksa= 13,518 Kg/m3 (Perrys Chemical engineering Handbook)
Massa jenis CO2= 1,8836 Kg/m3 (Perrys Chemical engineering Handbook)
E. PERHITUNGAN LAJU ALIR CO2
Variabel 1 (NaOH 0.2 N ; QNaOH = 3,3310-4 L/sec)
(
( )
(
(
Variabel 2 (NaOH 0.2 N ; QNaOH = 510-4 L/sec)
)
)
P1
( )
(
(
)
)
Variabel 3 (NaOH 0.2 N ; QNaOH = 6,6710-4 L/sec)
( )
(
(
)
)
F. PERHITUNGAN LAJU ALIR UDARA
Massa jenis raksa= 13,518 Kg/m3 (Perrys Chemical engineering Handbook)
Massa jenis O2= 1,24 Kg/m3 (Perrys Chemical engineering Handbook)
Variabel 1 (NaOH 0.2 N ; QNaOH = 3,3310-4 L/sec)
(
( )
(
(
)
)
P1
Variabel 2 (NaOH 0.2 N ; QNaOH = 510-4 L/sec)
( )
(
(
Variabel 3 (NaOH 0.2 N ; QNaOH = 6,6710-4 L/sec)
( )
(
(
G. PENENTUAN KADAR CO2 MULA MULA
Q2
Q1
Q3
NERACA TOTAL
Variabel 1 (NaOH 0.2 N ; QNaOH = 3,3310-4 L/sec)
Q1 = QCO2 =
)
)
P1
Q2 = Qudara =
Q3 = Q1 + Q2 = (
) L/s = 4,7868 L/s
Variabel 2 (NaOH 0.2 N ; QNaOH = 510-4 L/sec)
Q1 = QCO2 =
Q2 = Qudara =
Q3 = Q1 + Q2 = (
+ 1,9615) L/s = 2,3001 L/s
Variabel 3 (NaOH 0.2 N ; QNaOH = 6,6710-4 L/sec)
Q1 = QCO2 =
Q2 = Qudara =
Q3 = Q1 + Q2 = (
+ 1,9615) L/s = 2,3001 L/s
Neraca Komponen
C3 Q3 = C1 Q1 + C2 Q2
C3 = (C2 Q2)/ Q3
P.V = n.R.T
Tekanan CO2 dalam tabung
Variabel 1 (NaOH 0.2 N ; QNaOH = 3,3310-4 L/sec)
C2
C2
C3
C3
Variabel 2 (NaOH 0.2 N ; QNaOH = 510-4 L/sec)
C2
C2
C3
C3
Variabel 3 (NaOH 0.2 N ; QNaOH = 6,6710-4 L/sec)
C2
(C1=0)
P1
C2
C3
C3
H. Perhitungan hasil percobaan
Jika a > b, maka :
n Na2CO3
n NaHCO3
Jika a < b, maka :
n Na2CO3
n NaHCO3
n CO2 terserap = mol Na2CO3 + mol NaHCO3
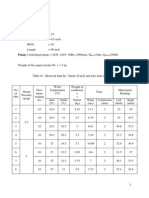
Variabel 1 (NaOH 0.2 N ; QNaOH = 3,3310-4 L/sec)
t menit
a ml
b ml
Na2CO3
NaHCO3
0
1
2
3
4
5
6
4
3.5
2.7
2
1.7
2.2
2.1
9.9
10.5
11.3
11.5
12.5
12.4
11.7
0.198
0.21
0.226
0.23
0.25
0.248
0.234
0.059
0.07
0.086
0.095
0.108
0.102
0.096
CO2
terserap
0.257
0.28
0.312
0.325
0.358
0.35
0.33
1.9
11.6
0.232
0.097
0.329
12.2
0.244
0.102
0.346
11.6
0.232
0.096
0.328
10
2
26.1
11.7
126.9
0.234
2.538
0.097
1.008
0.331
3.546
Variabel 2 (NaOH 0.2 N ; QNaOH = 510-4 L/sec)
t menit
a ml
b ml
Na2CO3
NaHCO3
CO2 terserap
5.7
0.16
0.023
0.183
P1
8.6
0.172
0.026
0.198
9.1
0.182
0.031
0.213
9.2
5.5
0.184
0.037
0.221
8.9
6.1
0.178
0.028
0.206
0.18
0.03
0.21
9.5
6.2
0.19
0.033
0.223
9.7
5.9
0.194
0.038
0.232
8.7
5.7
0.174
0.03
0.204
9.5
6.2
0.19
0.033
0.223
10
9.5
5.5
0.19
0.04
0.23
99.7
64.8
1.994
0.349
2.343
Variabel 3 (NaOH 0.2 N ; QNaOH = 6,6710-4 L/sec)
t menit
a ml
b ml
Na2CO3
NaHCO3
CO2 terserap
10.8
0.216
0.048
0.264
11
5.5
0.22
0.055
0.275
10.9
0.218
0.059
0.277
10.5
4.5
0.21
0.06
0.27
10
0.2
0.06
0.26
10
4.2
0.2
0.058
0.258
10.5
4.5
0.21
0.06
0.27
10.2
4.5
0.204
0.057
0.261
10.5
4.5
0.21
0.06
0.27
10.5
4.5
0.21
0.06
0.27
10
10.5
0.21
0.065
0.275
115.4
51.2
2.308
0.642
2.95
I. PERHITUNGAN HARGA Kga
RUMUS
P1
Dimana :
cm2
A = Luas kolom absorbsi =
Z = tinggi packing = 33 cm
= fraksi kosong = 0,7374
Variabel 1 (NaOH 0.2 N ; QNaOH = 3,3310-4 L/sec)
Variabel 2 (NaOH 0.2 N ; QNaOH = 510-4 L/sec)
Variabel 3 (NaOH 0.2 N ; QNaOH = 6,6710-4 L/sec)
J. PERHITUNGAN Kla
(
CO2= 1,8836 Kg/m3
= 0,7374
Variabel 1 (NaOH 0.2 N ; QNaOH = 3,3310-4 L/sec)
(
P1
284471794,9 dp1,4003
Variabel 2 (NaOH 0.2 N ; QNaOH = 510-4 L/sec)
(
Variabel 3 (NaOH 0.2 N ; QNaOH = 6,6710-4 L/sec)
(
P1
K. PERHITUNGAN k2
]
]
N NaOH = 0,2 N
g mole/cm3.atm
H=
P=
atm
Ra= QNaOH mol CO32[OH-]=
Variabel 1 (NaOH 0.2 N ; QNaOH = 3,3310-4 L/sec)
Ra = 3,3310-4 3.546 = 1,180810-3
P1
Variabel 2 (NaOH 0.2 N ; QNaOH = 510-4 L/sec)
Ra = 510-4 2.343 = 1,171510-3
Variabel 3 (NaOH 0.2 N ; QNaOH = 6,6710-4 L/sec)
Ra = 6,6710-4 2.95 = 1,967610-3
P1
You might also like
- Tripoli University Faculty of Engineering Chemical Engineering DepartmentDocument9 pagesTripoli University Faculty of Engineering Chemical Engineering DepartmentSrewaBenshebilNo ratings yet
- Determination of Mean Astivity Coefficient and Solubility of Potassium Hydrogen Tartrate (KHT) in Aqueous Solution at 30ºCDocument9 pagesDetermination of Mean Astivity Coefficient and Solubility of Potassium Hydrogen Tartrate (KHT) in Aqueous Solution at 30ºCAmeerul Hazeeq100% (10)
- Volumetirc Analysis Lab CsecDocument3 pagesVolumetirc Analysis Lab CsecGabriella SteeleNo ratings yet
- LEMBAR PERHITUNGAN FixDocument11 pagesLEMBAR PERHITUNGAN FixAhmad Andika HimawanNo ratings yet
- Laporan Absorpsi-1Document11 pagesLaporan Absorpsi-1FaqihudinMubarokNo ratings yet
- Lembar Perhitungan: N GR MR × V ×valensi GR × ×1Document16 pagesLembar Perhitungan: N GR MR × V ×valensi GR × ×1adelia10No ratings yet
- Mass Transfer in Packed ColumnDocument6 pagesMass Transfer in Packed ColumnthakkerNo ratings yet
- BIOGAS PurificationDocument4 pagesBIOGAS PurificationMeet KhuntNo ratings yet
- Coll PPDocument4 pagesColl PPRishi SinhaNo ratings yet
- Lampiran B PerhitunganDocument15 pagesLampiran B PerhitunganSundari PratiwiNo ratings yet
- Physical Chemistry: Answer KeyDocument18 pagesPhysical Chemistry: Answer Keyvishal110085No ratings yet
- Mole Concept Booklet SolutionDocument33 pagesMole Concept Booklet SolutionAkshay PatelNo ratings yet
- Gases and Gas LawsDocument6 pagesGases and Gas LawsMauricio Argel Ruíz CabañasNo ratings yet
- Lampiran A B C 5000Document586 pagesLampiran A B C 5000Muhammad Adam ANo ratings yet
- 5.3 Analisis Data 5.3.1 Tabel Data Pengamatan: Naoh NaohDocument10 pages5.3 Analisis Data 5.3.1 Tabel Data Pengamatan: Naoh NaohDode AnomNo ratings yet
- Chem 112.1 - Exer 9 Table and AnswersDocument7 pagesChem 112.1 - Exer 9 Table and AnswersGerry Mark GubantesNo ratings yet
- Answers To Topic 2 ExercisesDocument3 pagesAnswers To Topic 2 ExercisesgabriellaanastasiaNo ratings yet
- Observed Data Shell: Nominal Size 6: Max MaxDocument19 pagesObserved Data Shell: Nominal Size 6: Max MaxTanvir AhmedNo ratings yet
- Waste Heat BoilerDocument16 pagesWaste Heat Boilerdevilturn70No ratings yet
- Updating - MTO I - Unit 2 ProblemsDocument3 pagesUpdating - MTO I - Unit 2 ProblemsmaheshNo ratings yet
- CHEG 201 Chemical Process Calculation Homework #3Document11 pagesCHEG 201 Chemical Process Calculation Homework #3AASHISH CHAULAGAINNo ratings yet
- PB (No) + 2Kcl PBCL + 2kno: 2 (S) 2+ (Aq) - (Aq)Document14 pagesPB (No) + 2Kcl PBCL + 2kno: 2 (S) 2+ (Aq) - (Aq)yennioctaNo ratings yet
- Lampiran B Contoh PerhitunganDocument13 pagesLampiran B Contoh PerhitunganbambanggNo ratings yet
- Bangladesh University of Engineering and TechnologyDocument8 pagesBangladesh University of Engineering and TechnologyMd Abid AfridiNo ratings yet
- Preparing Standard Acid and BaseDocument7 pagesPreparing Standard Acid and Basebrittany obrienNo ratings yet
- D.1 Barium Klorida (Bacl) : Pelarut ODocument24 pagesD.1 Barium Klorida (Bacl) : Pelarut OSundari PratiwiNo ratings yet
- Dekanter (D-01) : Nacn Nacl C H CH CL C H CH CN H ODocument19 pagesDekanter (D-01) : Nacn Nacl C H CH CL C H CH CN H ORafi Theda PrabawaNo ratings yet
- Nov 2022 H2 Chemistry 9729 Paper 4 Suggested SolutionDocument20 pagesNov 2022 H2 Chemistry 9729 Paper 4 Suggested Solutionzavairling05No ratings yet
- Oxygen Determination in WaterDocument5 pagesOxygen Determination in Watermnazar.unsyiahNo ratings yet
- Eksekusi 1Document858 pagesEksekusi 1Faris HamidiNo ratings yet
- PPT AtkDocument10 pagesPPT AtkRyan WahyudiNo ratings yet
- ElektroDocument3 pagesElektroaprilia kurnia putriNo ratings yet
- Netralizer Koil JozDocument44 pagesNetralizer Koil JozPradika WibowoNo ratings yet
- Hydraulics: Lab 1 - Friction Losses in Pipe FlowDocument11 pagesHydraulics: Lab 1 - Friction Losses in Pipe FlowdilaNo ratings yet
- Lampiran B Perhitungan A. Perhitungan Untuk Tabel Kecepatan Volumetrik, Head Loss, DanDocument35 pagesLampiran B Perhitungan A. Perhitungan Untuk Tabel Kecepatan Volumetrik, Head Loss, Dannur irfana mardiyahNo ratings yet
- Tugas 4-Neraca Massa Dan Energi: Mixe R Reaktor Distilasi Prose S OverallDocument46 pagesTugas 4-Neraca Massa Dan Energi: Mixe R Reaktor Distilasi Prose S Overallpeter adityaNo ratings yet
- Mass BalanceDocument7 pagesMass BalancemahmoudNo ratings yet
- Acetic 2520acid 2520 - Design 2520of 2520equipments PDFDocument41 pagesAcetic 2520acid 2520 - Design 2520of 2520equipments PDFTanuj HandaNo ratings yet
- 010 2059 Prahasti Rombel 01 Tugas Ke 04Document14 pages010 2059 Prahasti Rombel 01 Tugas Ke 04prahasticynthiaNo ratings yet
- Ja5061388 Si 001Document38 pagesJa5061388 Si 001vasut.nakNo ratings yet
- Volume 15 cm×15,1 CM ×95 CM: Lembar Perhitungan 1. Volume ReaktorDocument6 pagesVolume 15 cm×15,1 CM ×95 CM: Lembar Perhitungan 1. Volume ReaktoryuiNo ratings yet
- Daftar Pustaka Dan LampiranDocument26 pagesDaftar Pustaka Dan LampiranWahyu TriNo ratings yet
- Solucoes ICHO28 A ICHO24Document38 pagesSolucoes ICHO28 A ICHO24Leonardo FagundesNo ratings yet
- Tutorial 1 AnswerDocument15 pagesTutorial 1 Answerd3kamsNo ratings yet
- Nitric AcidDocument14 pagesNitric Acidmalini2201No ratings yet
- 110 WS Gas Stoichiometry KeyDocument2 pages110 WS Gas Stoichiometry Keyエルミタ ジョイ ファティマ100% (1)
- 110 WS Gas Stoichiometry KeyDocument2 pages110 WS Gas Stoichiometry KeyDestiny Marie NavarroNo ratings yet
- Old AP Exam Gas Law Problems KeyDocument11 pagesOld AP Exam Gas Law Problems KeyJJNo ratings yet
- Solution: Part 1Document4 pagesSolution: Part 1Aljebre MohmedNo ratings yet
- ML ML ML N ML N ML N ML N: Appendix B Calculations & ComputationsDocument14 pagesML ML ML N ML N ML N ML N: Appendix B Calculations & ComputationshaanaNo ratings yet
- Lemper Hidrodinamika ReaktorDocument7 pagesLemper Hidrodinamika ReaktorAnindita IndrianaNo ratings yet
- 00 Perhitungan Alat Besar 1-DeSKTOP-NJGKKH8Document359 pages00 Perhitungan Alat Besar 1-DeSKTOP-NJGKKH8Muhammad FakhrizalNo ratings yet
- Lembar Perhitungan ABSDocument12 pagesLembar Perhitungan ABSNearya SondangNo ratings yet
- Basic Environmental Technology Water Supply Waste Management and Pollution Control 6th Edition Nathanson Test BankDocument38 pagesBasic Environmental Technology Water Supply Waste Management and Pollution Control 6th Edition Nathanson Test Bankmitchellunderwooda4p4d100% (13)
- Answers Practice Questions PDFDocument7 pagesAnswers Practice Questions PDFDlo Perera100% (1)
- V2 (m/s) V1 (m/s) P1 (Pa) P2 (Pa) Q (m^3/h) fasumido v (m/s) Re fcalculado L (m) D (m) z1 (m) z2 (m) ε (m) RR=ε/D μ (kg/ (m*s) ) g (m/s^2) ρ (kg/m3) γ (N/m3) ν (m2/s)Document4 pagesV2 (m/s) V1 (m/s) P1 (Pa) P2 (Pa) Q (m^3/h) fasumido v (m/s) Re fcalculado L (m) D (m) z1 (m) z2 (m) ε (m) RR=ε/D μ (kg/ (m*s) ) g (m/s^2) ρ (kg/m3) γ (N/m3) ν (m2/s)DiegoAlexNo ratings yet
- Burner (Repaired)Document21 pagesBurner (Repaired)Hengky FernandoNo ratings yet
- Problems: CHEM1020Document45 pagesProblems: CHEM1020Ahmed AliNo ratings yet
- Cutting-Edge Technology for Carbon Capture, Utilization, and StorageFrom EverandCutting-Edge Technology for Carbon Capture, Utilization, and StorageKarine Ballerat-BusserollesNo ratings yet
- Analytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportFrom EverandAnalytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportNo ratings yet
- Lembar PerhitunganDocument5 pagesLembar PerhitunganAnonymous a8990NENo ratings yet
- Lembar PerhitunganDocument8 pagesLembar PerhitunganAnonymous a8990NENo ratings yet
- Tugas Teknologi Pinch: No Nama Case Table TDocument1 pageTugas Teknologi Pinch: No Nama Case Table TAnonymous a8990NENo ratings yet
- Lembar Perhitungan PatiDocument5 pagesLembar Perhitungan PatiAnonymous a8990NENo ratings yet
- Lembar Perhitungan ReagenDocument12 pagesLembar Perhitungan ReagenAnonymous a8990NENo ratings yet
- Lembar Perhitungan ReagenDocument12 pagesLembar Perhitungan ReagenAnonymous a8990NENo ratings yet
- Lembar Perhitungan Reagen (Recovered)Document6 pagesLembar Perhitungan Reagen (Recovered)Anonymous a8990NENo ratings yet
- Lembar Perhitungan ReagenDocument3 pagesLembar Perhitungan ReagenAnonymous a8990NENo ratings yet
- Lembar Perhitungan Reagen (p3)Document6 pagesLembar Perhitungan Reagen (p3)Anonymous a8990NENo ratings yet