Professional Documents
Culture Documents
Carbon Dioxide Carbon Dioxide (
Uploaded by
iitsivaiahOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Carbon Dioxide Carbon Dioxide (
Uploaded by
iitsivaiahCopyright:
Available Formats
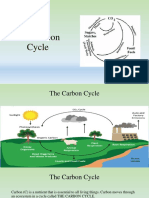
Carbon dioxide
Carbon dioxide (chemical formula CO2) is a naturally occurring chemical compound composed of
two oxygen atoms each covalently double bonded to a single carbon atom. It is a gas at standard
temperature and pressure and exists in Earth's atmosphere in this state, as a trace gas at a
concentration of 0.04 percent (400 ppm) by volume, as of 2014.[2]
As part of the carbon cycle, plants, algae, and cyanobacteria use light energy to photosynthesize
carbohydrate from carbon dioxide and water, with oxygen produced as a waste product.[3] However,
photosynthesis cannot occur in darkness and at night some carbon dioxide is produced by plants
during respiration.[4] It is produced during the respiration of all other aerobic organisms and is
exhaled in the breath of air-breathing land animals, including humans. Carbon dioxide is produced
during the processes of decay of organic materials and the fermentation of sugars in beer and
winemaking. It is produced by combustion of wood, carbohydrates and major carbon- and
hydrocarbon-rich fossil fuels such as coal, peat, petroleum and natural gas. It is emitted from
volcanoes, hot springs and geysers and is freed from carbonate rocks by dissolution in water and
acids. CO2 is found in lakes, at depth under the sea and commingled with oil and gas deposits.[5]
You might also like
- THE Carbon Cycle: Aiyna Ali & Jade LutchmanDocument6 pagesTHE Carbon Cycle: Aiyna Ali & Jade LutchmanjadeNo ratings yet
- carbon_lect4Document3 pagescarbon_lect4munna kumar vermaNo ratings yet
- Different Carbon ColorsDocument17 pagesDifferent Carbon ColorsKirushnananthy VallipuramNo ratings yet
- The Carbon CycleDocument3 pagesThe Carbon Cycleakeem babbNo ratings yet
- Jaglerod Mono-Di OksidDocument10 pagesJaglerod Mono-Di OksidLiljana DimeskaNo ratings yet
- Carbon Cycle PowerpointDocument17 pagesCarbon Cycle PowerpointJohn OsborneNo ratings yet
- Carbon CycleDocument17 pagesCarbon CycleJohn OsborneNo ratings yet
- Earth's AtmosphereDocument2 pagesEarth's AtmosphereEllee HadesNo ratings yet
- Biology 51 - 60Document8 pagesBiology 51 - 60ARWA SURVENo ratings yet
- Carbon CycleDocument6 pagesCarbon CycleHarrishNo ratings yet
- Carbon Dioxide: CarbohydrateDocument14 pagesCarbon Dioxide: CarbohydratePratik AgajNo ratings yet
- The Carbon Cycle: How Carbon Moves Through Plants, Animals and AtmosphereDocument6 pagesThe Carbon Cycle: How Carbon Moves Through Plants, Animals and AtmosphereCandy TuftNo ratings yet
- Sustainable Design Assign 1Document11 pagesSustainable Design Assign 1zohiiimughal286No ratings yet
- The Carbon, Water and Nitrogen Cycles ExplainedDocument8 pagesThe Carbon, Water and Nitrogen Cycles ExplainedKaz PianNo ratings yet
- CARBON COMPOUNDS: Pollution Aspects: Received Date: Jan. 2020 Revised: April 2020 Accepted: June 2020Document9 pagesCARBON COMPOUNDS: Pollution Aspects: Received Date: Jan. 2020 Revised: April 2020 Accepted: June 2020Vaibhav SiddharthNo ratings yet
- Terjemah Karya Tulis RIKADocument17 pagesTerjemah Karya Tulis RIKAKeyla AishaNo ratings yet
- Carbon Cycle ProcessesDocument17 pagesCarbon Cycle ProcessesKurniawan RizkiNo ratings yet
- Science ProjectDocument4 pagesScience ProjectNoor AbubakerNo ratings yet
- The Carbon Cycle HandoutDocument2 pagesThe Carbon Cycle HandoutthereseNo ratings yet
- Carbon Dioxide-Wps OfficeDocument2 pagesCarbon Dioxide-Wps OfficeMohd AimanNo ratings yet
- Chemistry Honors Project 3Document3 pagesChemistry Honors Project 3api-220385579No ratings yet
- The Carbon Cycle: CO2 Exchange Between Land, Sea & AirDocument2 pagesThe Carbon Cycle: CO2 Exchange Between Land, Sea & Airschoolworks IrishNo ratings yet
- The Carbon CycleDocument12 pagesThe Carbon CycleClariene CaburnayNo ratings yet
- TBI_co2_and_the_carbon_cycleDocument3 pagesTBI_co2_and_the_carbon_cyclepmcarqxnoogfbprtcfNo ratings yet
- lab 6 ADocument4 pageslab 6 Auzair akramNo ratings yet
- The Carbon Cycle ExplainedDocument10 pagesThe Carbon Cycle ExplainedFoisal SwarupNo ratings yet
- Environmental Chemistry (Air)Document32 pagesEnvironmental Chemistry (Air)Hussain HashmiNo ratings yet
- Carbon DioxideDocument4 pagesCarbon DioxidenontkawinkornNo ratings yet
- Biogeochemical CyclesDocument6 pagesBiogeochemical CyclesMaja LucasNo ratings yet
- Biogeochemical Cycle 2Document20 pagesBiogeochemical Cycle 2amodusofiat77No ratings yet
- Ecology 3 PDFDocument9 pagesEcology 3 PDFrasha nadaNo ratings yet
- Carbon Di Oxide and Carbon MonixideDocument1 pageCarbon Di Oxide and Carbon Monixideshaikh.ahmed.2117.eNo ratings yet
- Air Pollution-2021-22Document51 pagesAir Pollution-2021-22AshwiniNo ratings yet
- The Bad Effects of Vending Machine in EnvironmentDocument4 pagesThe Bad Effects of Vending Machine in Environmentaeso.does.gamingNo ratings yet
- Environmental ChemistryDocument17 pagesEnvironmental Chemistrytannie2512No ratings yet
- Enviromental IssuesDocument80 pagesEnviromental IssuesNAYAN BISWASNo ratings yet
- 4.3 Carbon CyclingDocument7 pages4.3 Carbon CyclingamalNo ratings yet
- Carbon CycleDocument14 pagesCarbon CycleRosano SelgaNo ratings yet
- The Carbon Cycle UpdatedDocument15 pagesThe Carbon Cycle UpdatedFoisal SwarupNo ratings yet
- Carbon DioxideDocument1 pageCarbon DioxideAlizay AamirNo ratings yet
- Air Pollution FinalDocument23 pagesAir Pollution Finalnamansehgal3006No ratings yet
- Concept of Air PollutionDocument74 pagesConcept of Air PollutionSaneet AgrawalNo ratings yet
- Biogeochemical CyclesDocument11 pagesBiogeochemical CyclesShipra agrawalNo ratings yet
- Carbon CycleDocument8 pagesCarbon CycleLenovoNo ratings yet
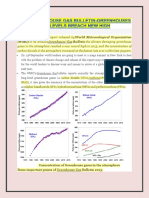
- Wmo Greenhouse Gas Bulletin-Greenhouses Gas Levels Breach New HighDocument5 pagesWmo Greenhouse Gas Bulletin-Greenhouses Gas Levels Breach New HighRamBabuMeenaNo ratings yet
- The Carbon CycleDocument12 pagesThe Carbon CycleClariene CaburnayNo ratings yet
- Products and Effects of CombustionDocument5 pagesProducts and Effects of CombustionLaura IonescuNo ratings yet
- Carbon Cycle: Submitted By, Jeeva Raj Joseph 1 M.Sc. M.B. MsrcascDocument24 pagesCarbon Cycle: Submitted By, Jeeva Raj Joseph 1 M.Sc. M.B. MsrcascSikhya PradhanNo ratings yet
- Environmental ChemistryDocument40 pagesEnvironmental ChemistryharryNo ratings yet
- Oxygen N Carbon CycleDocument12 pagesOxygen N Carbon CycleScience,Physical Education And Sports VideosNo ratings yet
- ES Lecture 6Document15 pagesES Lecture 6Erico TambanganNo ratings yet
- carbon cycle2Document2 pagescarbon cycle2The King studioNo ratings yet
- Air & Water PresentationDocument19 pagesAir & Water PresentationVictoria OlutimehinNo ratings yet
- Digital Assignment 1Document14 pagesDigital Assignment 1HariketNo ratings yet
- L-1 - Fate of Pollutants - Final (1) - 20Document21 pagesL-1 - Fate of Pollutants - Final (1) - 20Abdullah AkramNo ratings yet
- CHEMISTRY-AND-THE-ANTHROSPHEREDocument22 pagesCHEMISTRY-AND-THE-ANTHROSPHEREMichelle Dela CruzNo ratings yet
- Air Pollution Sources & EffectsDocument6 pagesAir Pollution Sources & Effectscory kurdapyaNo ratings yet
- Environmental Chemistry Chapter Provides Overview of Key ConceptsDocument9 pagesEnvironmental Chemistry Chapter Provides Overview of Key ConceptsHaa KksakNo ratings yet
- Carbon Cycle-File Grade 6Document4 pagesCarbon Cycle-File Grade 6Rasha GhabbounNo ratings yet
- Fun Facts about Carbon : Chemistry for Kids The Element Series | Children's Chemistry BooksFrom EverandFun Facts about Carbon : Chemistry for Kids The Element Series | Children's Chemistry BooksNo ratings yet
- Primary CareDocument1 pagePrimary CareiitsivaiahNo ratings yet
- Milad un-Nabi/Id-e-Milad in IndiaDocument3 pagesMilad un-Nabi/Id-e-Milad in IndiaiitsivaiahNo ratings yet
- Cyanocarbon Tear Gas Riot Control AgentDocument1 pageCyanocarbon Tear Gas Riot Control AgentiitsivaiahNo ratings yet
- Chemical Element Atomic Number Pnictogen Diatomic Universe Milky Way Solar System Earth's Atmosphere Daniel RutherfordDocument1 pageChemical Element Atomic Number Pnictogen Diatomic Universe Milky Way Solar System Earth's Atmosphere Daniel RutherfordiitsivaiahNo ratings yet
- Heterocyclic Organic Compound Chemical Formula C H N Benzene Methine Group Nitrogen Azines Niacin PyridoxalDocument1 pageHeterocyclic Organic Compound Chemical Formula C H N Benzene Methine Group Nitrogen Azines Niacin PyridoxaliitsivaiahNo ratings yet
- Primary CareDocument1 pagePrimary CareiitsivaiahNo ratings yet
- Hort Message Service (SMS) Is A Text Messaging Service Component of Phone, Web, orDocument1 pageHort Message Service (SMS) Is A Text Messaging Service Component of Phone, Web, oriitsivaiahNo ratings yet
- For A More Accessible and Less Technical Introduction To This TopicDocument4 pagesFor A More Accessible and Less Technical Introduction To This TopiciitsivaiahNo ratings yet
- Complex Numbers Part 1Document1 pageComplex Numbers Part 1iitsivaiahNo ratings yet
- Complex Number OperationsDocument1 pageComplex Number OperationsiitsivaiahNo ratings yet
- Tear GasDocument1 pageTear GasiitsivaiahNo ratings yet
- Cos Summative 3 EmblemDocument1 pageCos Summative 3 EmblemiitsivaiahNo ratings yet
- Conjugate of Complex Number Part4Document1 pageConjugate of Complex Number Part4iitsivaiahNo ratings yet
- Complex Numbers Part 1Document1 pageComplex Numbers Part 1iitsivaiahNo ratings yet
- Conjugate of Complex Number Part 2Document1 pageConjugate of Complex Number Part 2iitsivaiahNo ratings yet
- VII Physics111Document2 pagesVII Physics111iitsivaiahNo ratings yet
- Noble MetalsDocument1 pageNoble MetalsiitsivaiahNo ratings yet
- Physics Mass Velocity State of Rest Accelerate Magnitude Direction Vector SI Unit NewtonsDocument1 pagePhysics Mass Velocity State of Rest Accelerate Magnitude Direction Vector SI Unit NewtonsiitsivaiahNo ratings yet
- Electrical Conductor Circuit Semiconductor Electrolyte Vacuum William Whewell Michael Faraday Greek ElectricityDocument1 pageElectrical Conductor Circuit Semiconductor Electrolyte Vacuum William Whewell Michael Faraday Greek ElectricityiitsivaiahNo ratings yet
- Electron Configuration, Balancing Equations, Ions, and Isotopes QuizDocument1 pageElectron Configuration, Balancing Equations, Ions, and Isotopes QuiziitsivaiahNo ratings yet
- File ManagementDocument1 pageFile ManagementiitsivaiahNo ratings yet
- EthanolDocument1 pageEthanoliitsivaiahNo ratings yet
- Physics Mass Velocity State of Rest Accelerate Magnitude Direction Vector SI Unit NewtonsDocument1 pagePhysics Mass Velocity State of Rest Accelerate Magnitude Direction Vector SI Unit NewtonsiitsivaiahNo ratings yet
- DiodeDocument1 pageDiodeiitsivaiahNo ratings yet
- Latin Chemical Compound Oxide Silicon Chemical Formula Si O QuartzDocument1 pageLatin Chemical Compound Oxide Silicon Chemical Formula Si O QuartziitsivaiahNo ratings yet
- Physics Mass Velocity State of Rest Accelerate Magnitude Direction Vector SI Unit NewtonsDocument1 pagePhysics Mass Velocity State of Rest Accelerate Magnitude Direction Vector SI Unit NewtonsiitsivaiahNo ratings yet
- Anode and Cathode in Electrochemical CellsDocument1 pageAnode and Cathode in Electrochemical CellsiitsivaiahNo ratings yet
- Primary CellDocument1 pagePrimary CelliitsivaiahNo ratings yet
- HydroxideDocument1 pageHydroxideiitsivaiahNo ratings yet