Professional Documents
Culture Documents
Thermodynamic Tables SI 1s8 18
Uploaded by
KuroRick93Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Thermodynamic Tables SI 1s8 18
Uploaded by
KuroRick93Copyright:
Available Formats
60 0
h = 40
1800
kJ/
2200 kg
h=
2000
2400
2600
00
328200
3000
50%
20
00
3200
3400
4000
3800
3600
4500
22
00
Sa
tu
ra
0k
00
or
24
va
p
70
te d
0
10
80
0
80
0
60
0
50
0
40 0
35 00
3 0
25 0
20
0
15
60
h = 4200 kJ/
kg
5
Entropy, kJ/kg K
18
80 0
J/ k
g
20 0
26
00
k J/k
4200
4300
4400
4500
4600
4700
4800
4900
5000
h = 5000 kJ/kg
h=
80%
Qu
a lit
y=
90 %
2650
h = 2600
2650 kJ/kg
2550
2800
2900
3000
3100
3200
3300
3400
3500
3600
3700
3800
3900
4000
4100
0
10
100
200
300
400
500
600
700
800
900
1000
1100
10
1200
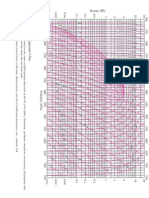
Copyright 1984. From NBS/NRC Steam Tables/1 by Lester Haar, John S. Gallagher, and George S. Kell. Reproduced by permission of Routledge/Taylor & Francis
Books, Inc.
%
20
1600
id
iqu
dl
ate
tur
Sa
1 00 0
60%
FIGURE A9
T-s diagram for water.
0%
=1
0
100
0
120
0
140
1 20
0
0
ty
ali
Qu
00
100
200
300
400
500
600
700
800
900
1000
Temperature, C
kg/m 3
Den
sity
=
50
00
10
00
1100
14
30
%
550
0
P
=
300
20 00 b
1 000 ar
5
100 000
80000
0
50
4 00 6000
30000
0
20 0
15 00
00
0
16
40%
300 k
g/m 3
100 k
40
g/m 3
30
2
0
15
30 kg
8
/m 3
10
6
4
10 kg
/m 3
3
2
1.5
3 kg/m 3
0.8
1
0.6
.0
0.4
1 kg/m 3
0.
3
0.2
0.3
0.1
kg/m 3
0.0
5
0.08 0.
1
6
0.1
0.
kg/m 3
0
0.04
3
0.0
0 2
.
0
15
0.0
3k
Den
0.0
g/m 3
s
i
0
t
y
8
=0
.01
kg/m 3
900
0.
0 006
0 .0
.
0 04
0. 03
00
2
0
1200
cen84959_ap01.qxd 4/27/05 2:59 PM Page 900
Thermodynamics
You might also like
- Question 1 (15 Marks)Document5 pagesQuestion 1 (15 Marks)Farouk BassaNo ratings yet
- Introduction To Chemical Processes Murphy Chapter06 SolutionsDocument94 pagesIntroduction To Chemical Processes Murphy Chapter06 SolutionsEric Barnett29% (7)
- Combustion of Pulverised Coal in a Mixture of Oxygen and Recycled Flue GasFrom EverandCombustion of Pulverised Coal in a Mixture of Oxygen and Recycled Flue GasNo ratings yet
- Steam/Condensate & Water Engineering DataDocument27 pagesSteam/Condensate & Water Engineering DataWalter SchamberNo ratings yet
- Chapter 14 CS-1Document7 pagesChapter 14 CS-1lionfierce12350% (2)
- 900 Termodinamik: 00 %% Ec Es IDocument1 page900 Termodinamik: 00 %% Ec Es IAlberto GutierrezNo ratings yet
- Property Tables Booklet Cengel SI Thermodynamics 6th Ed.Document1 pageProperty Tables Booklet Cengel SI Thermodynamics 6th Ed.MohamedJaberKutkutMjkNo ratings yet
- Zeroth Law: Ifaandbandbandcarein Thermal Equil, Then A and C Are in Thermal EquilDocument52 pagesZeroth Law: Ifaandbandbandcarein Thermal Equil, Then A and C Are in Thermal Equilkamal El NasharNo ratings yet
- Steam TablesDocument20 pagesSteam TablesFenny PutriNo ratings yet
- Diagrama - Del Vapor de Agua: Entalpía Específica (KJ/KG)Document1 pageDiagrama - Del Vapor de Agua: Entalpía Específica (KJ/KG)antoniojlopez16No ratings yet
- Standard Panel RadiatorsDocument4 pagesStandard Panel RadiatorsAlexandru VladimirNo ratings yet
- ZadacaDocument10 pagesZadacaMuamera HodzicNo ratings yet
- Thermodynamics Steam TableDocument10 pagesThermodynamics Steam Tablependrive80No ratings yet
- AhuDocument1 pageAhuckyprianou100% (1)
- Ope FinalDocument22 pagesOpe FinalAlvaro Boris Vallejos CortezNo ratings yet
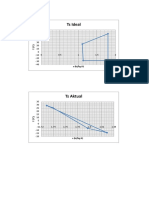
- Ts Ideal: S (KJ/KG.K)Document3 pagesTs Ideal: S (KJ/KG.K)Yunie Nthuw MitsukiNo ratings yet
- Revision SolutionDocument19 pagesRevision SolutionHassan Abo NagaNo ratings yet
- From Table 2-8 PerrysDocument2 pagesFrom Table 2-8 PerrysYasmin KayeNo ratings yet
- L V L M V: Design Equation: 1000 1Document3 pagesL V L M V: Design Equation: 1000 1solehah misniNo ratings yet
- Thermodynamic Steam Trap CatlogueDocument1 pageThermodynamic Steam Trap CatlogueJignesh BavlechaNo ratings yet
- Standards Steam Tables SI Compressed Liquid WaterDocument1 pageStandards Steam Tables SI Compressed Liquid WaterThiaNo ratings yet
- Thermo 5th Chap10 P067Document25 pagesThermo 5th Chap10 P067Ahmad AlgarniNo ratings yet
- HVAR - Exp4 - 191M028 - Varad RautDocument12 pagesHVAR - Exp4 - 191M028 - Varad Rautvarad rautNo ratings yet
- Exercise ResoSir GST PDF File XxxvVu4Document69 pagesExercise ResoSir GST PDF File XxxvVu4Abhinandan JainNo ratings yet
- Me 211 Examples SolutionsDocument30 pagesMe 211 Examples SolutionsBryan Dominic Gabriel PaduaNo ratings yet
- Tarefa 3 PTGDocument4 pagesTarefa 3 PTGGyano TrindadeNo ratings yet
- Example CH 4Document4 pagesExample CH 4Uday Prakash SahuNo ratings yet
- I P H S V M& T: I I I I I IDocument5 pagesI P H S V M& T: I I I I I IgowthamAG07No ratings yet
- Azaz Teknik KimiaDocument2 pagesAzaz Teknik KimiaExel Dua CincinNo ratings yet
- Ysis From The Steam Tables (Tables A-4, A-5, and A-6) ,: S S H H P TDocument11 pagesYsis From The Steam Tables (Tables A-4, A-5, and A-6) ,: S S H H P TEdison GuachambozaNo ratings yet
- CH 06Document76 pagesCH 06manthan21267% (3)
- Tugas AtkDocument5 pagesTugas AtklordzanigiNo ratings yet
- Pipe DiameterDocument1 pagePipe DiameternggothoNo ratings yet
- Evaporatoare Lu VeDocument3 pagesEvaporatoare Lu VeOtto OttoNo ratings yet
- PC - Lab 2 Q1&Q2Document4 pagesPC - Lab 2 Q1&Q2Priyanka NagpureNo ratings yet
- GweghewhDocument5 pagesGweghewhgowthamAG07No ratings yet
- Entalpija SimetricnaDocument270 pagesEntalpija SimetricnaMarcelo SouzaNo ratings yet
- Changing For Engg. MIS DataDocument4 pagesChanging For Engg. MIS DataANILNo ratings yet
- HW5Document6 pagesHW5YTK96No ratings yet
- CHNG 3802 Heat Transfer Tutorial Answers Weeks 1-4Document9 pagesCHNG 3802 Heat Transfer Tutorial Answers Weeks 1-4IshanSaneNo ratings yet
- Reheating Intercooling Combined ReferenceDocument12 pagesReheating Intercooling Combined ReferenceMatthew MeasNo ratings yet
- Danstoker Elkedel Datablad UK NOV22Document3 pagesDanstoker Elkedel Datablad UK NOV22mustafa subhiNo ratings yet
- Table TermodinamikaDocument24 pagesTable TermodinamikaAam Phobia MusisiNo ratings yet
- Eestudy Co UkDocument1 pageEestudy Co UkcataiceNo ratings yet
- Vle Report-Che19018Document3 pagesVle Report-Che19018Rathika RathikaNo ratings yet
- Lecture 3 - Steam Turbines (Sept 2020) PDFDocument9 pagesLecture 3 - Steam Turbines (Sept 2020) PDFrushdiNo ratings yet
- V: P 30 Inhg - 20 Inhg 10 Inhg or 0.0338639 MpaDocument4 pagesV: P 30 Inhg - 20 Inhg 10 Inhg or 0.0338639 MpaScrappy WellNo ratings yet
- Question 1. Liquid Water at 200 Kpa and 15: S S M S M T Q DT DsDocument6 pagesQuestion 1. Liquid Water at 200 Kpa and 15: S S M S M T Q DT Dsfivos_rgNo ratings yet
- Answers: 0.2467 1421.06kJ/kg 9.09kJ/kg 46%Document16 pagesAnswers: 0.2467 1421.06kJ/kg 9.09kJ/kg 46%Marianne DevillenaNo ratings yet
- Chapter 15Document4 pagesChapter 15Marco LuigiNo ratings yet
- Example 3C: Vapor Erheated Sup, Bar 40, C 500 at 5Document2 pagesExample 3C: Vapor Erheated Sup, Bar 40, C 500 at 5Daniel Camilo SalamancaNo ratings yet
- Thermo HWDocument6 pagesThermo HWMuhammad Fawwad ObaidaNo ratings yet
- HW#1Document7 pagesHW#1MahsaNo ratings yet
- Assignment 8Document8 pagesAssignment 8Jihan MutiahNo ratings yet
- Electricity in Fish Research and Management: Theory and PracticeFrom EverandElectricity in Fish Research and Management: Theory and PracticeNo ratings yet
- Total Energy: International Series in Heating, Ventilation and RefrigerationFrom EverandTotal Energy: International Series in Heating, Ventilation and RefrigerationNo ratings yet