Professional Documents
Culture Documents
Lab 2
Uploaded by
Matt PraterOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Lab 2
Uploaded by
Matt PraterCopyright:
Available Formats
Matt Prater
Lab #2
Quantitative NMR
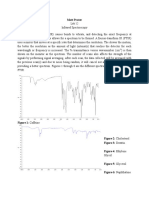
NMR instruments can be used to find the relative concentrations of different species in a
solution. This is particularly useful for analyzing organic solvents to find the ratio of
concentrations within the mixture. For this particular experiment, we had unknown #1 which was
a mixture of methanol and tert-butanol; and unknown #5 that consisted of ethanol and methanol.
Unknown #1 had a molar ratio of 2.59 : 1.00 (meOH : t-buOH) and mass percents of 52.8%
methanol and 47.2% tert-butanol. Unknown #5 had a molar ratio of 2.46 : 1.00 (etOH : meOH)
and mass percents of 77.6 % ethanol and 22.4% methanol. The NMR has the ability to produce a
very high accuracy in molar ratios. Table 1 summarizes the Data.
Table 1.
unkno
wn 1
methla
tnol
buOH
Molar Ratio
2.59
1
mass
%
52.8
47.2
unkno
wn 2
EtOH
MeOH
2.46
1
77.6
22.4
You might also like
- Refractive IndexDocument9 pagesRefractive IndexZirtaeb Cerdena0% (1)
- End Group 2Document22 pagesEnd Group 2Sabha Khalid shafiqNo ratings yet
- Extraction of Aromatics From Petroleum Naphtha Reformate by Solvent PDFDocument6 pagesExtraction of Aromatics From Petroleum Naphtha Reformate by Solvent PDFmehul10941100% (1)
- Partial Molar VolumeDocument4 pagesPartial Molar VolumeCorine CaracasNo ratings yet
- Determination of Intermolecular Interactions in Polar and Non-Polar Organic Molecules by Optical (Refractive Index) MethodDocument5 pagesDetermination of Intermolecular Interactions in Polar and Non-Polar Organic Molecules by Optical (Refractive Index) MethodchemistryjournalNo ratings yet
- Synthesis and Solution Properties of Poly (Monoethylphenyl Itaconate)Document3 pagesSynthesis and Solution Properties of Poly (Monoethylphenyl Itaconate)daniel burtonNo ratings yet
- Articulo MentolDocument4 pagesArticulo Mentoljuan_k5950No ratings yet
- Analytical Method of Propyl ParabenDocument7 pagesAnalytical Method of Propyl Parabenrusbianto wijayaNo ratings yet
- Lab Report Exp 1Document15 pagesLab Report Exp 1Justine Camille CastilloNo ratings yet
- Polymer Analysis Using NMRDocument2 pagesPolymer Analysis Using NMRMavia NaushadNo ratings yet
- 1 s2.0 0168365989900734 MainDocument7 pages1 s2.0 0168365989900734 MainJôsy SouzaNo ratings yet
- General Chemistry 1Document6 pagesGeneral Chemistry 1Sitti Rohima MarajanNo ratings yet
- Module 2 Lecture Part 1Document11 pagesModule 2 Lecture Part 1Princess joy De RuedaNo ratings yet
- Lesson 3. The Relationship of Percent Composition and Chemical FormulaDocument4 pagesLesson 3. The Relationship of Percent Composition and Chemical FormulaRandel MontielNo ratings yet
- Development and Validation of UV Spectrophotometric Method For Simultaneous Estimation of Metformin HCL and Repaglinide in Bilayer Tablet Dosage FormDocument6 pagesDevelopment and Validation of UV Spectrophotometric Method For Simultaneous Estimation of Metformin HCL and Repaglinide in Bilayer Tablet Dosage Formnish115862047No ratings yet
- Clo3 Reactions in Transesterification Process: E + S ES P + EDocument3 pagesClo3 Reactions in Transesterification Process: E + S ES P + ESyazwani AbdullahNo ratings yet
- A Validated UV Spectrophotometric Method For Simultaneous Estimation of Tretinoin and Benzoyl PeroxiDocument7 pagesA Validated UV Spectrophotometric Method For Simultaneous Estimation of Tretinoin and Benzoyl PeroxiJohnny DadaNo ratings yet
- Obtaining The Phenytoin and The InfluenceDocument10 pagesObtaining The Phenytoin and The InfluenceAylinNayeliNo ratings yet
- Jurnal Kimdas Modul 1Document3 pagesJurnal Kimdas Modul 107231038No ratings yet
- Chapter-4 RP-HPLC Method For Determination of Tapentadol and Its Related ImpuritiesDocument25 pagesChapter-4 RP-HPLC Method For Determination of Tapentadol and Its Related ImpuritiesRavi YadavNo ratings yet
- Metodologia de AnalisisDocument10 pagesMetodologia de AnalisisHéctor Fabio Leyton ArcosNo ratings yet
- Miyake 1998Document5 pagesMiyake 1998JessicaNo ratings yet
- The Effect of Ether On The Reaction Rate of Mtbe SynthesisDocument7 pagesThe Effect of Ether On The Reaction Rate of Mtbe SynthesisEryl YeongNo ratings yet
- J. Org. Chem. 2012, 77, 5198-5202 (Rotamer Diastereoisomer NMR)Document5 pagesJ. Org. Chem. 2012, 77, 5198-5202 (Rotamer Diastereoisomer NMR)ludoNo ratings yet
- Mean Centering of Ratio Spectra Method For The Analysis of Theophylline and Ephedrine HCL Mixture in TabletDocument6 pagesMean Centering of Ratio Spectra Method For The Analysis of Theophylline and Ephedrine HCL Mixture in TabletGita SupyanaNo ratings yet
- JPSR 03110401Document5 pagesJPSR 03110401Cypriano NetoNo ratings yet
- Thermodynamic Study On Density and Viscosity of Binary Mixtures of Ethyl Acetoacetate With (C4-C9) Aliphatic Ketones at (303.15 and 308.15) KDocument17 pagesThermodynamic Study On Density and Viscosity of Binary Mixtures of Ethyl Acetoacetate With (C4-C9) Aliphatic Ketones at (303.15 and 308.15) KInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- BioRes 10 2 2691 Sarinana TFES Simple Method Determ Bioeth Prod Coffee 6710Document8 pagesBioRes 10 2 2691 Sarinana TFES Simple Method Determ Bioeth Prod Coffee 6710sameenamehtabNo ratings yet
- RSC Adv., 2014, 4, 61022-61027 Enzymatic Activity SIDocument38 pagesRSC Adv., 2014, 4, 61022-61027 Enzymatic Activity SIVeronicaIguarbeMontalbanNo ratings yet
- Science: Percentage Composition of CompoundsDocument16 pagesScience: Percentage Composition of CompoundsAnnie Bagalacsa Cepe-Teodoro100% (1)
- ScipaperExp4 Group1Document6 pagesScipaperExp4 Group1Kim Moscosa100% (1)
- Research Article: Validation of A UV Spectrometric Method For The Assay of Tolfenamic Acid in Organic SolventsDocument19 pagesResearch Article: Validation of A UV Spectrometric Method For The Assay of Tolfenamic Acid in Organic SolventsAni Yunita SariNo ratings yet
- Antecedentes 2Document26 pagesAntecedentes 2Carlos Mario Ortiz MuñozNo ratings yet
- Chemesthesis: Chemical Touch in Food and EatingFrom EverandChemesthesis: Chemical Touch in Food and EatingShane T. McDonaldNo ratings yet
- Results and Discussion: 3.1 Extraction YieldDocument3 pagesResults and Discussion: 3.1 Extraction YieldYuliastri DewiNo ratings yet
- Two Step Aprotic Solvent Catalyzed Deikmann Condensation of Phenethylamine and MethylacrylateDocument7 pagesTwo Step Aprotic Solvent Catalyzed Deikmann Condensation of Phenethylamine and MethylacrylateTravis BoltNo ratings yet
- Acoustical Studies of Drug Combiflam in Aqueous Alcoholic MixturesDocument5 pagesAcoustical Studies of Drug Combiflam in Aqueous Alcoholic MixturesSEP-PublisherNo ratings yet
- INFORME Lab Termo 2Document5 pagesINFORME Lab Termo 2Emily Tatiana Alvarez VillaNo ratings yet
- Results and Discussion (3.1-3.2)Document6 pagesResults and Discussion (3.1-3.2)Dhanesh KumarNo ratings yet
- Application of ERAS-model and Prigogine-Flory-Patterson Theory To ExcessDocument8 pagesApplication of ERAS-model and Prigogine-Flory-Patterson Theory To ExcessMario Ricardo Urdaneta ParraNo ratings yet
- (20835736 - Acta Chromatographica) HPLC Method For Simultaneous Determination of Metronidazole and Preservatives in Vaginal Gel FormulationDocument4 pages(20835736 - Acta Chromatographica) HPLC Method For Simultaneous Determination of Metronidazole and Preservatives in Vaginal Gel FormulationArtem KulikovNo ratings yet
- C I E B: Ommon ON Ffect and UffersDocument1 pageC I E B: Ommon ON Ffect and UffersDoom RefugeNo ratings yet
- HSGC ValidationDocument6 pagesHSGC Validation9704609609No ratings yet
- Experiment 4: Common-Ion Effect and Buffers Ignatius Dominic P. Cumigad College of ScienceDocument2 pagesExperiment 4: Common-Ion Effect and Buffers Ignatius Dominic P. Cumigad College of ScienceJemimahNo ratings yet
- N, N'-Azobis-Isobutyronitrile (AIBN) As The Initiator. Addition To All of These Mainly Properties ofDocument7 pagesN, N'-Azobis-Isobutyronitrile (AIBN) As The Initiator. Addition To All of These Mainly Properties ofAoNo ratings yet
- Method Development and Validation For The Estimation of Metronidazole in Tablet Dosage Form by UV Spectroscopy and Derivative SpectrosDocument5 pagesMethod Development and Validation For The Estimation of Metronidazole in Tablet Dosage Form by UV Spectroscopy and Derivative SpectrosSriram NagarajanNo ratings yet
- Batch Extractive Distillation of Mixture Methanol-Acetonitrile Using Aniline As A AsolventDocument6 pagesBatch Extractive Distillation of Mixture Methanol-Acetonitrile Using Aniline As A AsolventBharat SharmaNo ratings yet
- Optimasi EKstraksi KarotenoidDocument9 pagesOptimasi EKstraksi KarotenoidpatophoriaNo ratings yet
- Luluk Pratiwi, Muhammad Saifur Rachman, Nur IdayatiDocument7 pagesLuluk Pratiwi, Muhammad Saifur Rachman, Nur IdayatiNinis RahayuNo ratings yet
- Review of Methods For The Analisys of Protein HidrolisatesDocument9 pagesReview of Methods For The Analisys of Protein HidrolisatesDaniel Dávila MartinezNo ratings yet
- Park 2002Document11 pagesPark 2002Juan Camilo AgudeloNo ratings yet
- HPLC Research PaperDocument19 pagesHPLC Research PaperMit PatelNo ratings yet
- Figueiras 2010Document8 pagesFigueiras 2010kamakaballestas87No ratings yet
- Lecture 7Document21 pagesLecture 7dineshkumaryadav39423No ratings yet
- Invitro Quantification of Flavonoids and Phenolic Content of - SuranDocument5 pagesInvitro Quantification of Flavonoids and Phenolic Content of - SuranJimoh Daud SmartNo ratings yet
- Direct Enantioseparation of Adrenergic Drugs Via Thin-LayerDocument9 pagesDirect Enantioseparation of Adrenergic Drugs Via Thin-Layerq52rqhqsybNo ratings yet
- Lornoxicam (Jurnal Pendukung)Document5 pagesLornoxicam (Jurnal Pendukung)farizfaturNo ratings yet
- Comparison of HPLC and GC-MS Methods For Determination of Embutramide (A Component of Tanax - or T-61 - in Biological SpecimensDocument5 pagesComparison of HPLC and GC-MS Methods For Determination of Embutramide (A Component of Tanax - or T-61 - in Biological SpecimensADAMNo ratings yet
- Matt Prater: Tds MG Solids Liters SolutionDocument4 pagesMatt Prater: Tds MG Solids Liters SolutionMatt PraterNo ratings yet
- Lab 5Document1 pageLab 5Matt PraterNo ratings yet
- Lab 6Document6 pagesLab 6Matt PraterNo ratings yet
- Lab 12Document2 pagesLab 12Matt PraterNo ratings yet
- Table 1: Retention Factors at An Eluent Strength of 10Document1 pageTable 1: Retention Factors at An Eluent Strength of 10Matt PraterNo ratings yet
- Lab 4Document4 pagesLab 4Matt PraterNo ratings yet
- Matt Prater Lab 3Document4 pagesMatt Prater Lab 3Matt PraterNo ratings yet
- Ethylenediamine Complexes of ChromiumDocument5 pagesEthylenediamine Complexes of ChromiumMatt PraterNo ratings yet
- Iron Sulfur Clusters For DNA RepairDocument3 pagesIron Sulfur Clusters For DNA RepairMatt PraterNo ratings yet
- Figure 1. Four Common Chelating AgentsDocument3 pagesFigure 1. Four Common Chelating AgentsMatt PraterNo ratings yet
- Qualitative NMR (#1) : Matt Prater Group 4 Analysis LabDocument1 pageQualitative NMR (#1) : Matt Prater Group 4 Analysis LabMatt PraterNo ratings yet
- Theory of SolubilityDocument17 pagesTheory of SolubilityAmeen ShahidNo ratings yet
- Solution Digital Notes by Bharat SirDocument16 pagesSolution Digital Notes by Bharat SirKartik SharmaNo ratings yet
- Electrochemistry Worksheet.Document3 pagesElectrochemistry Worksheet.talha parvezNo ratings yet
- Chapter8-Campuran Pada Tingkat Molekuler - Part 1Document58 pagesChapter8-Campuran Pada Tingkat Molekuler - Part 1Uswatun KhasanahNo ratings yet
- ICH Q3C Residual SolventsDocument40 pagesICH Q3C Residual SolventsRiccardo TorelliNo ratings yet
- Worksheet3 48489Document2 pagesWorksheet3 48489Naga AshokNo ratings yet
- Lakshya Jee 2023: SolutionDocument4 pagesLakshya Jee 2023: SolutionHarshit ChaudharyNo ratings yet
- Solutions NotesDocument34 pagesSolutions NotesAneesh RenuNo ratings yet
- WK 7 SolubilityDocument26 pagesWK 7 SolubilitychrizNo ratings yet
- Factors Affecting SolubilityDocument35 pagesFactors Affecting SolubilityJescil Ann OriolNo ratings yet
- CBSE Class 12 Chemistry - Solutions AssignmentDocument15 pagesCBSE Class 12 Chemistry - Solutions AssignmentPoonam TripathiNo ratings yet
- Xii Chemistry - CH 02 - Solutions - Question BankDocument12 pagesXii Chemistry - CH 02 - Solutions - Question BankBUNNY GOUD100% (1)
- Grade 7 Science Solubility ExperimentDocument5 pagesGrade 7 Science Solubility Experimentapi-21257927975% (8)
- USP 42 Description & Relative SolubilityDocument85 pagesUSP 42 Description & Relative Solubilityirhamfauzzi100% (3)
- SolutionsDocument17 pagesSolutionsNamita SinhaNo ratings yet
- Solubility Prediction of Salicylic Acid in Water-Ethanol-Propylene GlycolDocument4 pagesSolubility Prediction of Salicylic Acid in Water-Ethanol-Propylene GlycolChilaNo ratings yet
- Saturated and Unsaturated Solutions: Sci-BoxDocument9 pagesSaturated and Unsaturated Solutions: Sci-BoxNhet Ytienza50% (2)
- Properties of SolutionsDocument74 pagesProperties of SolutionsLamisa Imam50% (2)
- Chapter 01 Properties of SolutionDocument70 pagesChapter 01 Properties of SolutionYo Liang SikNo ratings yet
- Colligative PropertiesDocument26 pagesColligative PropertiesYAWAR SAEED100% (1)
- Colligative Properties of Nonelectrolyte SolutionsDocument40 pagesColligative Properties of Nonelectrolyte Solutionspogi69100% (5)
- SolutionDocument23 pagesSolutionrosariopraveen007No ratings yet
- Solutions Notes PDFDocument21 pagesSolutions Notes PDFSMELLY CATNo ratings yet
- Evaporation RateDocument2 pagesEvaporation RateLê TiếnNo ratings yet
- Ideal and Non-Ideal SolutionsDocument3 pagesIdeal and Non-Ideal SolutionsJamshaid AliNo ratings yet
- Alims - Approved Combined Terminology - December 2014Document5 pagesAlims - Approved Combined Terminology - December 2014Katarina R.M.No ratings yet
- H X Diagram Ammonia WaterDocument1 pageH X Diagram Ammonia Water蒲俊雄No ratings yet
- Las Melc 1 Week 3Document11 pagesLas Melc 1 Week 3Evelyn AndosonNo ratings yet
- 2 Solutions 97cDocument66 pages2 Solutions 97cRubiNo ratings yet
- 9094S1TKCE50332018 - Operasi Teknik Kimia II - Pertemuan 8 - TugasDocument1 page9094S1TKCE50332018 - Operasi Teknik Kimia II - Pertemuan 8 - Tugaslintang cahyaniNo ratings yet