Professional Documents
Culture Documents
Turbidimetric Determination of Sulfate
Uploaded by
Matt PraterOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Turbidimetric Determination of Sulfate
Uploaded by
Matt PraterCopyright:
Available Formats
Matt Prater
Lab 6
Turbidimetic determination of sulfate
Introduction
Turbidimetry is an analytical technique that uses the loss of intensity due to light being
scattered by particles that are present. The more particles present the more light gets scattered
and this causes intensity to be reduced as it hits the detector. This reduction in light intensity is
directly proportional with the amount of precipitate in the sample. A calibration curve was
established using values of 5 ppm through 40 ppm (by 5 ppm) sulfate concentrations in order to
form a linear curve that relates concentration of sulfate to Formazin Turbidity Unit and vice
versa. Once a calibration curve was established, the unknown #1 and the coal creek sample were
tested.
Procedure
Materials:
Magnetic stir bar/plate
Turbidimeter
Stop watch (x2)
Condition reagent (prepared by
Cameron)
BaCl2 crystals
Standard sulfate solution (100 ppm)
Distilled Water
Measuring Spoon
Following the preparation of the ~100 ppm standard by Madi Day, small volumes
of this solution were taken and diluted to form 5, 10, 15,,40 ppm standards using 5, 10,
15,, 40 mL of the standard and diluting to 100 mL. The solution for the 35 ppm
standard was diluted just above the calibrated line and therefore would be slightly below
the standard curve. The coal creek sample was diluted by 4 and the unknown was diluted
by 10 in order to use the turbidimeter. To each sample, 5 mL condition reagent which was
prepared by Cameron was added and then the 2-3 mL of BaCl2 crystals was added to
form a precipitate during one minute of stirring. While the first sample was stirring, a
blank was analyzed with the turbidimeter. Following the 1 minute of stirring, part of the
sample was poured into the sample vial and placed in the turbidimeter for a 1 minute
analysis. This occurred for the 8 standard solutions along with the two unknown samples.
Following the analysis, the calculations to relate concentration to the turbidity unit were
performed as in figure 1 below. One of the unknowns was a sample from the river at the
location in figure 2 below.
turbidity intercept
=concentration
slope
Figure 1: relation of turbidity to concentration.
Figure 2: Sampling location.
Results
The turbidimeter produced the following values: 0.51, 0.81, 1.08, 1.32, 1.55, 1.82,
2.01, and 2.40 for the concentrations of 5, 10, 15,, 40 respectively which formed a very
linear curve with an R2 value of 0.9961. These values are in table 1, and were used to
produce figure 3. The value from the turbidimeter for the unknown was 1.91 and the
value for the river sample was 1.88.
Calibration Curve
300
250
200
turbidity (unitless)
150
f(x) = 5.16x + 27.61
R = 1
Calibration Curve
Linear Regression
100
50
0
0 1020304050
Concentration (ppm)
Table 1: calibration data.
con
cent
rati
on
(pp
m)
5
10
15
20
25
30
T
u
r
b
i
d
i
t
y
5
1
8
1
1
0
8
1
3
2
1
5
5
1
8
2
35
40
2
0
1
2
4
0
Figure 3: Calibration curve.
The values from the river in order from the top of the creek to the bottom for
sulfate concentration were 130, 144, 129, 124, and 141 ppm with an average of 134 ppm
and a standard deviation of 8 ppm. These values appear in table 2. The unknown #1
concentration was 316.5 ppm.
Table 2: Sulfate river concentrations.
Location
rustys
canyon
park
lions
park
main
street
bridge
the Y
Unknow
n #1
Average
(river)
Stdev
(river)
Concentrat
ion (ppm)
130.3
144
129
124.3
141.4
316.5
133.8
8.4755530
79
Conclusion
The above values for the river concentrations all represent potable water due to a
maximum contamination for sulfate being 500 ppm.1 Due to a standard deviation of only
8 ppm, the values reported from the groups all agree to a pretty large extent. The river
sulfate concentration was 133.8 ppm is well within normal values and is very drinkable.
Turbidity as a method is very consistent yet the results were different than those of the
gravimetric analysis. This could be due to the difference in which day the water was
sampled and the time of day as well. The weather has been warming up and thus the ice
has been melting up higher; causing the flow rate to be higher and not solvate as many
ions in its travels. Therefore the concentration being lower makes sense.
References
(1)
USGS - NAWQA - Water Quality in the Allegheny and Monongahela River
Basins - Major Findings http://pubs.usgs.gov/circ/circ1202/major_findings.htm (accessed
Feb 2, 2015).
You might also like
- Technical Analysis Laboratory ManualDocument54 pagesTechnical Analysis Laboratory ManualP P SELVI selvi.chemNo ratings yet
- Flame Photometry AnalysisDocument6 pagesFlame Photometry AnalysisleonardoNo ratings yet
- Lab Report 2Document8 pagesLab Report 2api-296431001No ratings yet
- Water For InjectionsDocument4 pagesWater For InjectionsAlvina Arum PuspitasariNo ratings yet
- Solubility of Ionic Salts in Seawater (Experiment 4) : AbstractDocument7 pagesSolubility of Ionic Salts in Seawater (Experiment 4) : AbstractfizaNo ratings yet
- Determination of Hardness of Water and WastewaterDocument4 pagesDetermination of Hardness of Water and WastewaterThato NkhemeNo ratings yet
- Lab Report Environmental Engineering 2 (CEL304)Document40 pagesLab Report Environmental Engineering 2 (CEL304)Shivang KumarNo ratings yet
- Lab 5 Physical and Chemical Tests of A Mud Contaminated With Calcium ChloriteDocument9 pagesLab 5 Physical and Chemical Tests of A Mud Contaminated With Calcium Chloritealan713No ratings yet
- Experiment 6Document5 pagesExperiment 6Annam AsgharNo ratings yet
- Water Tests PupilDocument14 pagesWater Tests PupilJAMES CURRANNo ratings yet
- Commercial Antacid Neutralisation CapacityDocument9 pagesCommercial Antacid Neutralisation CapacityAbg Khairul Hannan Bin Abg AbdillahNo ratings yet
- Chlorides and SulphatesDocument4 pagesChlorides and SulphatesRESHMYNo ratings yet
- Lab Manual For IT (16 TH Dec)Document52 pagesLab Manual For IT (16 TH Dec)aattishNo ratings yet
- Chemistry SBA Long Report (Permanganate Index)Document13 pagesChemistry SBA Long Report (Permanganate Index)Matthew ChuNo ratings yet
- Determination of Lead in River Water by Flame Atomic Absorption SpectrometryDocument8 pagesDetermination of Lead in River Water by Flame Atomic Absorption SpectrometryputriNo ratings yet
- Exp 9Document7 pagesExp 9rachzammit2003No ratings yet
- Watertreatmentsimulationandanalysis EllismcnicholDocument5 pagesWatertreatmentsimulationandanalysis Ellismcnicholapi-302400368No ratings yet
- Water Analysis PrtocolDocument29 pagesWater Analysis PrtocolRajSharmaNo ratings yet
- Thin Layer ChromatographyDocument2 pagesThin Layer ChromatographyOdessa Vidallon100% (3)
- Lab ManualDocument19 pagesLab Manualanon_467104036No ratings yet
- Lab Report Environmental Engineering 2 (CEL304)Document40 pagesLab Report Environmental Engineering 2 (CEL304)Shivang KumarNo ratings yet
- Chlorides & AlkalinityDocument8 pagesChlorides & AlkalinityVohinh NgoNo ratings yet
- Acidity, Total and Calcium HardnessDocument6 pagesAcidity, Total and Calcium HardnessGaniyu JosephNo ratings yet
- Turbidimetri Sulfat PDFDocument2 pagesTurbidimetri Sulfat PDFSasmi NopiyaniNo ratings yet
- Lbych29 HandoutDocument24 pagesLbych29 HandoutKyle LatayanNo ratings yet
- Cyanide Leaching of GoldDocument11 pagesCyanide Leaching of GoldAzizul HakimNo ratings yet
- The Determination of Antimony, Tin and LeadDocument7 pagesThe Determination of Antimony, Tin and LeadSoledad ColmenarezNo ratings yet
- Lab Report (Final Editied)Document8 pagesLab Report (Final Editied)Alexia Channer100% (4)
- KMnO4 LabDocument4 pagesKMnO4 LabJevaughn SmallNo ratings yet
- CHEM1100 Experiment 5 Laboratory Report: Introduction: State The Purpose of The Experiment and BackgroundDocument3 pagesCHEM1100 Experiment 5 Laboratory Report: Introduction: State The Purpose of The Experiment and Backgroundemz_woxleyNo ratings yet
- Analytical Method of Mercury, Arsenic, Cadmium, Lead and Chromium in FertilizersDocument8 pagesAnalytical Method of Mercury, Arsenic, Cadmium, Lead and Chromium in FertilizersGenaro PalacioNo ratings yet
- Formal Report 1 Expt 7 Chem 26.1Document6 pagesFormal Report 1 Expt 7 Chem 26.1Franz Valencia100% (1)
- Candy Lab ReportDocument6 pagesCandy Lab ReportZhi Yang LinNo ratings yet
- SM+3500+Mn 20th+edDocument3 pagesSM+3500+Mn 20th+edNguyen KieuNo ratings yet
- Detect Hexavalent Chromium in WaterDocument5 pagesDetect Hexavalent Chromium in Wateralexaoo100% (3)
- Identification of An Unknown Organic Compound-1Document11 pagesIdentification of An Unknown Organic Compound-1Myrna Casden0% (1)
- Application of DC and Mark-Space Bias Differential Electrolytic Potentiometry For Determination of Cyanide Using A Programmable Syringe PumpDocument6 pagesApplication of DC and Mark-Space Bias Differential Electrolytic Potentiometry For Determination of Cyanide Using A Programmable Syringe PumpBhisma DamarekaNo ratings yet
- Determination of Water HardnessDocument5 pagesDetermination of Water HardnessLi Kim100% (1)
- Atomic Absorption Spectrometry Lab Report Experiment 06Document7 pagesAtomic Absorption Spectrometry Lab Report Experiment 06PDPPPMAT0621 Ruhilin Binti Nasser100% (1)
- EXPT 3 ChloridesDocument4 pagesEXPT 3 ChloridesReshmy M RajuNo ratings yet
- Engineering Chemistry Lab Manual PDFDocument25 pagesEngineering Chemistry Lab Manual PDFmayukrijuNo ratings yet
- Karl FischerDocument6 pagesKarl FischerRavi MawaleNo ratings yet
- Organic Carbon Test MethodDocument5 pagesOrganic Carbon Test MethodMPK08No ratings yet
- Determina Metal in Soil Bby MehlishDocument3 pagesDetermina Metal in Soil Bby MehlishPurna PirdausNo ratings yet
- No 3Document12 pagesNo 3Punit Ratna ShakyaNo ratings yet
- Chem 113 - Water Sample Lab ReportDocument16 pagesChem 113 - Water Sample Lab Reportapi-356033847No ratings yet
- Oiler Feed Water TestDocument7 pagesOiler Feed Water TestRISHIKESH KUMARNo ratings yet
- Fluoride PDFDocument7 pagesFluoride PDFevin34No ratings yet
- 3500-Ca Calcium 3500-Ca A.: 500 Mg/l. The Most CommonDocument2 pages3500-Ca Calcium 3500-Ca A.: 500 Mg/l. The Most CommonElard Vilca CutimboNo ratings yet
- Exp 1 Discussion and ConclusionDocument2 pagesExp 1 Discussion and ConclusionMarybeth HopeNo ratings yet
- Matt Prater Lab 3Document4 pagesMatt Prater Lab 3Matt PraterNo ratings yet
- Chem Lab #17Document6 pagesChem Lab #17NekuMinhNo ratings yet
- Advanced Pharmaceutical analysisFrom EverandAdvanced Pharmaceutical analysisRating: 4.5 out of 5 stars4.5/5 (2)
- Critical Evaluation of Some Equilibrium Constants Involving Alkylammonium Extractants: Commission on Equilibrium DataFrom EverandCritical Evaluation of Some Equilibrium Constants Involving Alkylammonium Extractants: Commission on Equilibrium DataNo ratings yet
- The Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresFrom EverandThe Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresRating: 5 out of 5 stars5/5 (1)
- Fourth International Conference on Non-Aqueous Solutions: Vienna 1974From EverandFourth International Conference on Non-Aqueous Solutions: Vienna 1974V. GutmannNo ratings yet
- Practical Manual of Analytical ChemistryFrom EverandPractical Manual of Analytical ChemistryRating: 4.5 out of 5 stars4.5/5 (3)
- Lab9 10Document15 pagesLab9 10Matt PraterNo ratings yet
- Lab 5Document1 pageLab 5Matt PraterNo ratings yet
- Alkalinity Determination Lab ReportDocument4 pagesAlkalinity Determination Lab ReportMatt PraterNo ratings yet
- Matt Prater: Tds MG Solids Liters SolutionDocument4 pagesMatt Prater: Tds MG Solids Liters SolutionMatt PraterNo ratings yet
- Lab 8Document1 pageLab 8Matt PraterNo ratings yet
- Lab 12Document2 pagesLab 12Matt PraterNo ratings yet
- Effect of Eluent Strength on Ion Chromatography RetentionDocument1 pageEffect of Eluent Strength on Ion Chromatography RetentionMatt PraterNo ratings yet
- Matt Prater Lab 3Document4 pagesMatt Prater Lab 3Matt PraterNo ratings yet
- Lab 18Document1 pageLab 18Matt PraterNo ratings yet
- NMR Analysis of Three Unknown SamplesDocument1 pageNMR Analysis of Three Unknown SamplesMatt PraterNo ratings yet
- AA LabDocument2 pagesAA LabMatt PraterNo ratings yet
- Unkno Wn1 Unkno Wn2: Methla Nol T-Buoh Etoh Meoh Molar Ratio 2.59 1 2.46 1 Mass % 52.8 47.2 77.6 22.4Document1 pageUnkno Wn1 Unkno Wn2: Methla Nol T-Buoh Etoh Meoh Molar Ratio 2.59 1 2.46 1 Mass % 52.8 47.2 77.6 22.4Matt PraterNo ratings yet
- Lab 11Document4 pagesLab 11Matt PraterNo ratings yet
- Alkalinity Determination Lab ReportDocument4 pagesAlkalinity Determination Lab ReportMatt PraterNo ratings yet
- Chelation Therapy Removes Toxins Like Mercury, Lead & CadmiumDocument3 pagesChelation Therapy Removes Toxins Like Mercury, Lead & CadmiumMatt PraterNo ratings yet
- Matt Prater Lab 3Document4 pagesMatt Prater Lab 3Matt PraterNo ratings yet
- Kinetics ReportDocument6 pagesKinetics ReportMatt PraterNo ratings yet
- Iron Sulfur Clusters For DNA RepairDocument3 pagesIron Sulfur Clusters For DNA RepairMatt PraterNo ratings yet
- Computational LabDocument1 pageComputational LabMatt PraterNo ratings yet
- NMR Analysis of Three Unknown SamplesDocument1 pageNMR Analysis of Three Unknown SamplesMatt PraterNo ratings yet
- Ethylenediamine Complexes of Chromium SpectraDocument5 pagesEthylenediamine Complexes of Chromium SpectraMatt PraterNo ratings yet
- Unkno Wn1 Unkno Wn2: Methla Nol T-Buoh Etoh Meoh Molar Ratio 2.59 1 2.46 1 Mass % 52.8 47.2 77.6 22.4Document1 pageUnkno Wn1 Unkno Wn2: Methla Nol T-Buoh Etoh Meoh Molar Ratio 2.59 1 2.46 1 Mass % 52.8 47.2 77.6 22.4Matt PraterNo ratings yet
- Exp KidneyDocument5 pagesExp KidneyMariam RahimNo ratings yet
- Staal 40: Gate ValvesDocument8 pagesStaal 40: Gate ValvesEric LarrondoNo ratings yet
- Bubble Point and Dew Point Calculations University of The Philippines DilimanDocument7 pagesBubble Point and Dew Point Calculations University of The Philippines DilimanAcademicBMNo ratings yet
- A Comparative Pharmaceutico-Analytical Study of Punarnasava and PunarnarishtaDocument5 pagesA Comparative Pharmaceutico-Analytical Study of Punarnasava and Punarnarishtaalnrmamckoppa19No ratings yet
- The Main Steps of The Manufacturing Process: Synthetic Rubber GlovesDocument3 pagesThe Main Steps of The Manufacturing Process: Synthetic Rubber GlovesLEE LEE LAUNo ratings yet
- Guggenheim 1935Document57 pagesGuggenheim 1935Niraj ThakreNo ratings yet
- TP-AATM 106b-A Moisture Karl Fischer MethodDocument2 pagesTP-AATM 106b-A Moisture Karl Fischer MethodRafael LugoNo ratings yet
- New Phytologist - 2022 - Prasad - Double DJ 1 Domain Containing Arabidopsis DJ 1D Is A Robust Macromolecule DeglycaseDocument14 pagesNew Phytologist - 2022 - Prasad - Double DJ 1 Domain Containing Arabidopsis DJ 1D Is A Robust Macromolecule DeglycaseSahilu RabiluNo ratings yet
- Monday 4 May 2020: BiologyDocument20 pagesMonday 4 May 2020: Biologycye23No ratings yet
- Electrochemical Energy Storage Enables RenewablesDocument37 pagesElectrochemical Energy Storage Enables RenewablesIvan Liviu IonNo ratings yet
- Kinetics of The Synthesis of Bisphenol A: Applied Catalysis, 37Document10 pagesKinetics of The Synthesis of Bisphenol A: Applied Catalysis, 37khalid -No ratings yet
- Clinical Chemistry (Electrolytes)Document27 pagesClinical Chemistry (Electrolytes)2B SALVADOR Jamaica C.No ratings yet
- SRB & TCB Tests EvaluationDocument25 pagesSRB & TCB Tests EvaluationEmad BehdadNo ratings yet
- Individuality and personality traitsDocument18 pagesIndividuality and personality traitsERNANIE LLAVORENo ratings yet
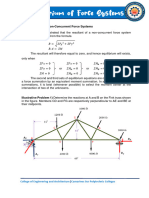
- Chapter 4. Equilibrium of Non Concurrent Force SystemsDocument7 pagesChapter 4. Equilibrium of Non Concurrent Force SystemscarminavegamariaNo ratings yet
- Aldec 45 - Manual - 2012 - enDocument134 pagesAldec 45 - Manual - 2012 - enCentrifugal SeparatorNo ratings yet
- Pharmaceutics 13 01313Document23 pagesPharmaceutics 13 01313maizhafiraNo ratings yet
- Chemistry Project Class 12Document12 pagesChemistry Project Class 12mihir khabiyaNo ratings yet
- Experiment 5abDocument9 pagesExperiment 5abapi-579557092No ratings yet
- Excipient Table 2Document4 pagesExcipient Table 2Marian OpreaNo ratings yet
- Driscal-D Polymer QMAXDocument1 pageDriscal-D Polymer QMAXAnonymous vSLFJCNNo ratings yet
- DETC2018-86042: Review of Natural Fiber Reinforced Elastomer CompositesDocument11 pagesDETC2018-86042: Review of Natural Fiber Reinforced Elastomer CompositesSanjayvadamudulaNo ratings yet
- Measurement of Cation Exchange Capacity (CEC) On Natural Zeolite by Percolation MethodDocument8 pagesMeasurement of Cation Exchange Capacity (CEC) On Natural Zeolite by Percolation MethodBayu WiyantokoNo ratings yet
- Journal of King Saud University - Engineering SciencesDocument8 pagesJournal of King Saud University - Engineering SciencesRodrigo ParedesNo ratings yet
- Topic 9Document28 pagesTopic 9deema dmdNo ratings yet
- Material and Energy Efficient Busbar Systems in Zinc and Copper PlantsDocument17 pagesMaterial and Energy Efficient Busbar Systems in Zinc and Copper PlantsAngelito_HBKNo ratings yet
- Myths Human GeneticsDocument72 pagesMyths Human GeneticsfukusacoNo ratings yet
- ACID TITLESDocument41 pagesACID TITLESAditi MishraNo ratings yet
- Topic 4 Chemical Bonding and Structure PDFDocument13 pagesTopic 4 Chemical Bonding and Structure PDFSveta StepanovaNo ratings yet
- Thermal Process Information BookDocument15 pagesThermal Process Information Book45 Aadhya RoyNo ratings yet