General Chemistry II Sample Test bank
1. According to the BronstedLowry definition,
which chemical species can function both as an
acid and as a base?
a. Cl
b. SO42
c. NH4+
d. HCO3
e. H3O+
2. In the reaction CN + H2O !" HCN + OH
which is an acidbase conjugate pair?
a. H2O and HCN
b. H2O and OH
c. CN and H2O
d. HCN and OH
3. Given that HX is a stronger Bronsted acid than
HY in aqueous solution, which is true of a 1 M
solution of NaX
a. It is less basic than a 1 M solution of NaY.
b. It is more basic than a 1 M solution of NaY.
c. It yields a neutral solution.
d. It is more concentrated than a 1 M solution
of NaY.
4. The pH of a 0.03 M HCl solution is
a. 1.5
b. 2.5
c. 3.5
d. 12.5
5. The pH of a 1.0 x 103 M Ba(OH)2 solution at
25oC is
a. 2.7
b. 3.0
c. 11.0
d. 11.3
6. Which is the strongest acid?
a.
b.
c.
d.
HClO
HClO2
HClO3
HClO4
7. Which salt reacts with water (hydrolyzes) to
produce a basic solution?
a.
b.
c.
d.
NaC2H3O2
NaNO3
NH4Cl
BaSO4
Page 1of 18
8. The weakest of the bases listed is
Acid
HCl
HSO4
H2S
HS
a. Cl
b. CN
c. HS
d. S2
e. SO42
Conjugate
Base
Cl
SO42
HS
S2
Ka (Ionization
Constant of Acid)
100% ionized
1.2 x 10-2
5.7x10-8
1.2x10-13
9. The oxide of which element will react with water
to form the strongest acid?
Period
First
Second
Third
a.
b.
c.
d.
e.
Main Groups
I
II II IV
I
X
W
VI
VII
Q
R
S
T
U
M
W
M
P
R
Z
10.
Which statement is a logical inference from
the fact that a 0.10 M solution of potassium
acetate KC2H3O2, is less alkaline than a 0.10 M
solution of potassium cyanide, KCN?
a.
b.
c.
d.
Hydrocyanic acid is a weaker acid than
acetic acid.
Hydrocyanic acid is less soluble in water
than acetic acid.
Cyanides are less soluble than acetates
Acetic acid is a weaker acid than
hydrocyanic acid
11.
In the titration of 50.0 mL of 0.100 M
benzoic acid (a monoprotic acid) with 50.0 mL of
0.100 M NaOH, the properties of the solution at
the equivalence point will correspond exactly to
the properties of
a. a 0.100 M sodium solution.
b. a 0.0500 M sodium hydroxide solution.
c. a 0.0500 M benzoic acid solution.
d. a 0.0500 M sodium benzoate solution.
�General Chemistry II Sample Test bank
Page 2of 18
12.
A mixture of which pair of 0.1 M aqueous
solutions would constitute a buffer?
a. NaOH and NaCl
b. HCl and NaOH
c. HCl and NaCl
d. NH3 and NH4NO3
13.
What do these have in common?
24
Ne 19F1Mg2+
20
a.
b.
c.
d.
the same number of protons
the same number of neutrons
the same number of electrons
the same size
14.
The number of neutrons in the nucleus of
an atom of 1327Al is
a. 40
b. 13
c. 27
d. 14
e. 9
15.
The orbitals of 2p electrons are often
represented as being
a. elliptical.
b. Pyramidal
c. Tetrahedral
d. dumbbell shaped
e. spherical.
16.
The element in Period 5, Group 3A, has the
outer electron configuration
a. 5s25p1
b. 3s23p5
c. 3s23p3
d. 5s25p3
17.
Which
electron
impossible?
a. 1s22s22p63s2
b. 1s22s22p63s23p6
c. 1s22s22p62d2
d. 1s22s22p53s1
configuration
is
18.
The element X occurs naturally to the
extent of 20.0% 12X and 80.0% 13X. The atomic
mass of X is nearest
a.
b.
c.
d.
12.2
12.5
12.8
13.0
19.
Which electron transition is associated with
the largest emission of energy?
a.
b.
c.
d.
n=2 to n=1
n=2 to n=4
n=2 to n=3
n=3 to n=2
20.
If an electron moves from one energy level
in an atom to another energy level more remote
from the nucleus of the same atom
a. energy is absorbed.
b. energy is liberated.
c. there is no energy change.
d. the atom must assume a different ionic
valence
e. light of a definite wave length is emitted.
21.
A photon of light of 450 nm, when
compared to light of wavelength 300 nm, has
(1nm = 10-9 m)
a.
b.
c.
d.
a higher frequency.
lower energy.
a greater velocity.
a shorter wavelength
22.
Which set of quantum numbers is possible
for an electron in an atom?
a.
b.
c.
d.
n=3, l=0, ml=1, ms = -
n=2, l=2,ml= -2, ms = -
n=5, l=2, ml=2, ms =+
n=4, l=3, ml= -4, ms= -
23.
A compound consisting of an element
having a low ionization potential and a second
element having a high electron affinity is likely
to have
a.
b.
c.
d.
covalent bonds
metallic bonds
coordinate covalent bonds
ionic bonds.
24.
According to modern bonding theory the
number of sigma () and pi () bonds in the
ethylene molecule H2C=CH2 is
a. l and 4
b. l and 1
c. l and 5
d. 2 and 4
e. l and 6
�General Chemistry II Sample Test bank
25.
a.
b.
c.
d.
The number of bonds in N=N is
1
2
3
4
26.
The elements in an ionic compound are
held together by
a.
b.
c.
d.
e.
electrostatic forces of attraction.
van der Waals forces
the spin of paired electrons.
the formation of hybrid orbitals.
an electron pair.
27.
In every electrolytic and galvanic (voltaic)
cell the anode is that electrode
a.
b.
c.
d.
at which oxidation occurs.
which attracts cations.
at which electrons are supplied to the
solution.
at which reduction occurs.
28.
Metal X was plated from a solution
containing cations of X. The passage of 48.25 C
deposited 31 mg of X on the cathode. What is the
mass of X (in grams) per mole of electrons?
a. 47
b. 62
c. 93
d. 186
29.
In a galvanic (voltaic) cell in which the
reaction is
Cd + Cu2+ " Cu + Cd2+
and the ions are at unit concentration (activity),
the cell potential is
Cd " Cd2++ 2eCu " Cu2+ + 2ea. 0.1383 V
b. 0.4021 V
c. 0.344 V
d. 0.7461 V
e. 0.3677 V
0.4021 V
- 0.344 V
30.
In which reaction will an increase in total
pressure at constant temperature favor formation
of the products?
a. CaCO3(s) !" CaO(s) + CO2(g)
b. H2(g) + Cl2(g) !"
" 2HCl(g)
c. 2NO(g) + O2(g) !" 2NO2(g)
d. COCl2(g) !" CO(g) + Cl2(g)
Page 3of 18
Standard Potentials
Eo
Mg" Mg2++2e
2.37V
Al " Al3++3e
1.66V
Zn " Zn2++2e
0.76V
2+
Fe"Fe +2e
0.44V
Cu " Cu2++2e
0.34V
Ag" Ag++ e
-0.80V
31.
Using only the metals Mg, Al, Zn, Fe, Cu
and Ag, together with their 1 M salt solutions, a
voltaic cell of the highest possible voltage would
be constructed using electrodes of these metals.
a. Mg and Ag
b. Mg and Fe
c. Zn and Cu
d. Al and Ag
e. Mg and Al
32.
E = Eo - 0.059/n log Q (Nernst equation)
+
[H ] = 1.0 M initially, P02 = 1.0 atm
4e + O2(g)+4H+(aq)!" 2H2O(l)
Eo=1.23V
Based on the information above, which statement
is correct?
a.
b.
c.
d.
n = 1, since one mole of oxygen is being
considered.
Addition of base should result in an E
value, which is less than 1.23 V.
E is independent of the pH of the solution.
Q = [H2O]2
[O2] [H+]
33.
The equilibrium constant for the gaseous
reaction C + D !" E + 2F is 3.0 at 50 oC. In a
2.0 L flask at 50 oC are placed 1.0 mol of C, 1.0
mol of D, 1.0 mol of E, and 3.0 mol of F.
Initially, the reaction will
a. proceed at equal rates in both directions.
b. proceed more rapidly to form E and F.
c. proceed more rapidly to form C and D.
d. not occur in either direction.
Compound Gof kJ/mol
H2O(l)
237
H2O(g)
229
34.
At 298 K the equilibrium constant for
H2(g) + O2(g)!" H2O(l)
a.
b.
c.
is larger than the Keq for
H2(g) + O2(g)!" H2O(g)
will have a value of 1 0 at equilibrium.
cannot be computed since data on O2 and
�General Chemistry II Sample Test bank
d.
H2 are not provided.
will have the same value as the Keq for
2 H2(g) + O2(g)!" 2 H2O(l)
35.
Consider the reversible system at
equilibrium:
2CO + O2 !" 2CO2 + heat
When the temperature is increased at constant
pressure
a.
b.
c.
d.
e.
the CO2 concentration will be increased.
the CO2 concentration will be decreased.
the amount of each substance will be
unchanged.
the amount of each substance will be
increased.
the result cannot be predicted from the
information given.
36.
The numerical value of the equilibrium
constant for any chemical change is affected by
changing
a. the catalyst.
b. the concentration of the products.
c. the concentration of reacting substances.
d. the pressure.
e. the temperature
37.
What is the equilibrium constant expression
for the gas phase oxidation of CO to CO2 by O2?
a.
K = [CO2]2
[CO][O2]
b.
K= [CO]2 [O2]
[CO2]
c.
K= [CO2]2
[CO]2 [O2]
d.
K= [CO] [O2]
[CO2]
38.
Into an empty vessel COCl2(g) is
introduced at 1.0 atm pressure whereupon it
dissociates until equilibrium is established:
2COCl2(g) !" C(graphite) +CO2(g) + 2Cl2(g)
a.
If x represents the partial pressure of
CO2(g) at equilibrium, what is the value of
the equilibrium constant, Kp?
x.2x2
(1.0-2x)2
Page 4of 18
b.
c.
d.
x x 2x2
(1.0-2x2)
x (2x)2
(1.0- 2x)2
x (2x)2
(1.0x)2
39.
At a certain temperature, the equilibrium
constant for the reaction 2HI(g) !"
" H2(g) +
I2(g) is 0.49. Calculate the number of moles of
hydrogen produced when one mole of HI is
placed in a 1 L vessel at this temperature.
a. 0.41
b. 0.25
c. 0.29
d. 3.45
40.
What is the [OH-] of a solution which is
0.18 M in ammonium ion and 0.10 M in
ammonia? Kb = 1.8 x 10-5
a. 1.3x10-3
b. 1.0x10-3
c. 1.3x10-5
d. 1.0x10-5
41.
What is the pH of a 0.10 M solution of a
monoprotic acid, HA, with a Ka = 1.0 x 10-6?
a. 1.6
b. 3.5
c. 5.0
d. 6.0
42.
When 0.10 mol of a weak acid HA was
diluted to one liter, experiment showed the acid
to be 1% dissociated.
HA + H2O !" H3O+ + AWhat is the acid dissociation constant, Ka?
a. 1 x10-6
b. 1 x10-5
c. l x10-3
d. 1 x105
43.
Which solution has a pH less than 7.0?
a. 1 M NH4Cl
b. 1 M K2CO3
c. 1 M NaOCl
d. 1 M NaOH
44.
What is the pH of a 0.1 M NaF solution?
Ionization constant for HF, Ka = 7 x 10-4
a. 2.1
b. 5.9
c. 8.1
d. 9.1
�General Chemistry II Sample Test bank
45.
What is the hydrogen ion concentration of a
buffer solution containing 0.10 M NO21- and 0.20
M HNO2? Ionization constant for Nitrous
Acid, Ka = 4.5x 10-4
a.
b.
c.
d.
2.2x10-4M
9.0 x 10-4 M
4.5 x 10-4 M
9.5 x 10-3M
46.
The solubility of BaCO3 is 7.9 x 10-3 g/L.
Calculate the solubility product, Ksp ignoring
hydrolysis. MW of BaCO3 197 g/mol
a.
b.
c.
d.
1.6x10-2
1.6x10-9
4.0x10-5
6.2x10-5
47.
The addition of solid Na2SO4 to an aqueous
solution in equilibrium with solid BaSO4 will
cause
a.
b.
c.
d.
no change in [Ba2+] in solution.
more BaSO4 to dissolve.
precipitation of more BaSO4.
an increase in the Ksp of BaSO4.
48.
Assume that standardized aqueous solutions
of each of these are available.
Substance Ionization
Constant
NaOAc
Kb = 5.6 x 10-10
RNH3+Cl- Ka = 5.6 x 10-10
RNH2
Kb=1.8x10-5
HOAc
Ka=1.8x10-5
A buffer with a desired pH is 5.0 would be
conveniently prepared by appropriate mixtures of
a. NaOAc and HOAc
b. HOAc and water
c. NaOAc and RNH2
d. HOAc and RNH2
49.
a.
b.
c.
d.
Which substance is most soluble in water?
C6H6
CaCO3
C2H5OH
CO2
50.
The solubility of BaCrO4 in water is 2.8 x
-3
10 g/L what is the Ksp of the salt? MW of
Page 5of 18
BaCrO4 253 g/mol
a. 1.2x10-10
b. 2.2x10-5
c. 2.8x10-3
d. 7.8 xl0-6
51.
Which is the correct expression for the
solubility product constant for Ag2CrO4?
a. Ksp = [Ag+]2 [CrO42-]
b. Ksp = [2Ag+]2 [CrO42-]
c. Ksp = [Ag+] [CrO42-]
d. Ksp = [Ag+] [CrO42-]2
e. Ksp = [2Ag+] [CrO42-]
52.
a.
b.
c.
d.
The correct IUPAC name of N2O3 is
nitrogen oxide.
nitrogen(II) oxide.
nitrous oxide
dinitrogen trioxide.
53.
In which case is the substance with the
given formula followed by its correct name?
a. KNO2 potassium nitrate
b. FeCl3 iron(III) chloride
c. FeS iron(II) sulfite
d. Mg3N2 magnesium nitrite
e. HClO hydrochloric oxide
54.
Balance the equation for the following
reaction, using no fractional coefficients.
? C +? HNO3 " ? CO2 +? NO2 +? H2O
The sum of the coefficients in the balanced
equation is
a. 5
b. 7
c. 9
d. 12
e. 16
55.
Complete and balance the equation for the
reaction, where the reactants are in aqueous
solution. Use no fractional coefficients.
? Na3PO4 + ? Ba(NO3)2 " ? +
The number of moles and formula of the product
containing Ba are
a. 3NaNO3
b. BaPO4
c. Ba(PO4)2
d. Ba2P3
e. Ba3(PO4)2
�General Chemistry II Sample Test bank
56.
According to the kinetic molecular theory,
a.
b.
c.
d.
e.
gaseous molecules are continuously in
random motion and collisions are perfectly
elastic.
the absolute temperature of a gas depends
on its molar mass.
the pressure exerted on a gas affects the
speed of its molecules.
gaseous molecules can travel in straight or
curved paths.
all gaseous molecules are diatomic.
57.
The volume of a given mass of gas varies
inversely with pressure, provided that the
temperature remains constant because
a.
b.
c.
d.
e.
attractive forces between gas molecules are
negligible.
attractive forces between gas molecules are
appreciable.
the average kinetic energy of the molecules
of a gas is proportional to the absolute
temperature.
increasing the molecular concentration, at
constant temperature, means increasing the
number of collisions between molecules
and container.
collisions between gas molecules are
perfectly elastic.
58.
The Kelvin temperature of one liter of gas
is doubled and its pressure is tripled, volume will
then be
l
/6 L
/3 L
3
/2 L
6L
59.
Which gas, present in the same closed
system, has the greatest average kinetic energy at
a given temperature?
a.
b.
c.
d.
a.
b.
c.
d.
Page 6of 18
60.
A sample of neon occupies a volume of
27.3 L at STP. What would be the neon volume
at 177 oC and 0.100 atm pressure?
a.
b.
c.
d.
177 L
350 L
422 L
450 L
61.
Under the same conditions of temperature
and pressure, the gas whose molecules possess
the highest average speed is
a. H2O
b. O2
c. F2
d. Ne
62.
What is the volume of 2.00 mol of helium
gas at 27 oC and 3.00 atm?
a.
b.
c.
d.
63.
6.1x10-2L
1.48L
16.4L
44.8 L
Real gases are most like ideal gases at
a.
b.
c.
d.
high pressure and high temperature.
low pressure and low temperature.
high pressure and low temperature.
low pressure and high temperature.
64.
500 mL of a gaseous compound has a mass
of 0.9825 g at 0oC and 760 mmHg. What is the
approximate molar mass of the compound?
a. 19.7
b. 38.7
c. 44.0
d. 58.9
65.
The partial pressures of a gaseous mixture
are given in the table. What is the mole percent
of hydrogen?
Hydrogen
neon
carbon dioxide
None; the average kinetic energy is the
same for each gas.
a.
b.
c.
d.
hydrogen
carbon dioxide
methane
ethylene
20.0
25.8
38.8
41.7
Partial Pressures
200 mmHg
150 mmHg
320 mmHg
105 mmHg
�General Chemistry II Sample Test bank
66.
It is desired to collect enough oxygen over
water at 25 oC and 750 mmHg barometric
pressure to be equivalent to 1 L of pure oxygen at
0 oC and 760 mmHg. The vapor pressure of water
at 25 oC is 23.5 mm. The volume collected is
a. 1 L (298/273)(750/760)
b. 1 L (273/298)((750-23.5)/(760-23.5))
c. 1 L (273/298)((750-23.5)/760)
d. 1 L (298/273)(760/(750-23.5))
67.
Methane, CH4, diffuses in a given apparatus
at the rate of 30 mL/min. At what rate would a
gas with a molar mass of 100 diffuse under the
same conditions? MW of CH4 = 16 g/mol
a. 0.77 mL/min
b. 30 mL/min
c. 6.7 mL/min
d. 75 mL/min
e. 12.0 mL/min
68.
The Arrhenius equation, k= Ae-E/RT
expresses the relationship between the reaction
rate constant, k, and the energy of activation, E.
The probability that colliding molecules will
react
a. increases with increasing energy of
activation.
b. depends only on the empirical constant, A.
c. increases with decreasing temperature.
d. decreases with increasing energy of
activation.
69.
The rate law for the reaction A+B"C+D is
first order in [A] and second order in [B]. If [A]
is halved and [B] is doubled, the rate of the
reaction will
a.
b.
c.
d.
remain the same
be increased by a factor of 2.
be increased by a factor of 4.
be increased by a factor of 8.
70.
The addition of a catalyst in a chemical
reaction
a. increases the concentration of products at
equilibrium.
b. increases the fraction of reactant molecules
with a given kinetic energy.
c. provides an alternate path with a different
activation energy.
d. lowers the enthalpy change in the overall
reaction.
Page 7of 18
71.
The following mechanism has been
proposed for the formation of ethylbenzene:
CH3CH2Br + AlBr3 " AlBr4- + CH3CH2+
CH3CH2+ + C6H6 " C6H6CH2CH3+
C6H6CH2CH3+ + AlBr4- "AlBr3 + HBr +
C6H5CH2CH3
Which substance serves as the catalyst?
a.
b.
c.
d.
AlBr3
CH3CH2+
AlBr4C6H6CH2CH3+
72.
Which substance has the highest boiling
point?
a.
b.
c.
d.
CH4
He
HF
Cl2
73.
The table presents data for the reaction: The
temperature of the reaction is constant. The initial
rate is in arbitrary units.
2H2(g) + 2NO(g) " 2H2O(g) + N2(g)
Exp.
I
II
III
IV
Initial Concentration (mol/L)
[NO] x 10-3 [H2] x 10-3 Initial Rate
6.0
1.0
18
6.0
2.0
36
1.0
6.0
3
2.0
6.0
12
What is the rate law for this reaction?
a. rate = k1 [H2][NO]
b. rate = k1 [H2]2 [NO]2
c. rate = k1 [H2]2 [NO]
d. rate = k1 [H2] [NO]2
74.
The reaction 2A + 2B " C +D proceeds by
this mechanism:
2A !" A2 (equilibrium)
A2 + B " X + C (rate determining)
X+B"D
(rapid)
The rate equation for the reaction is
a.
b.
c.
d.
rate = k[A] [B]
rate = k[A]2 [B]2/[C][D]
rate = k[A]2 [B]2[D]
rate = k[A]2 [B]
�General Chemistry II Sample Test bank
Page 8of 18
75.
What is the correctly reported mass of
water based on this data?
a.
Mass of beaker and water 29.62 g
Mass of beaker only 28.3220 g
a.
b.
c.
d.
1.3g
1.30g
1.298g
1.2980g
76.
When a small single piece of magnesium
ribbon is dropped into a test tube half filled with
dilute sulfuric acid, the metal soon floats to the
surface of the liquid. The best explanation for
this is that, (Densities, Mg, 1.79 g/cm3;
H2SO4(dilute) 1.2 g/cm3)
a.
b.
c.
d.
e.
the metal is less dense than the acid.
the metal gets hot and expands and
decreases its density markedly as it reacts
with the acid
the magnesium sulfate formed increases the
density of the solution.
gas bubbles attached to the metal buoy the
metal to the top.
convection currents set up in the acid carry
the metal to the top.
77.
The equilibrium vapor pressure of a few
liters of a liquid is dependent on
a.
b.
c.
d.
c.
d.
e.
80.
The stronger the intermolecular forces in a
substance
a.
b.
c.
d.
65.4
92.0
184
238
79.
When a hypothetical ionic crystal M+X- is
heated, it vaporizes to form separate M+(g) and
X-(g) ions. The energy required for this
vaporization (the lattice energy) will be greatest
when
the higher the boiling point.
the lower the boiling point.
the higher the vapor pressure.
the smaller the deviation from ideal gas
behavior.
81.
Which group of substances is correctly
arranged in order from the highest to the lowest
melting point?
a.
b.
c.
d.
HF>H2>NaF
NaF>H2>HF
HF>NaF>H2
NaF>HF>H2
82.
The fact that H2O has a dipole moment
suggests that the water molecule is
the mass of the liquid.
the surface area of the liquid
the temperature only
the volume of the liquid
78.
The edge of a unit cube of an element Y,
containing two atoms per unit cube, was found
(by X- ray diffraction) to be 3.16 x 10-8 cm. The
density of the metal is 19.35 g/cm3. What is the
approximate atomic molar mass of Y?
a.
b.
c.
d.
b.
the electron affinity of X is small in
magnitude and the ionization potential of M
is large in magnitude.
the heat of vaporization of crystalline M is
small.
the heat of vaporization of crystalline M is
large.
the effective radii of M+ and X- are large.
the effective radii of M+ and X- are small.
a.
b.
c.
d.
83.
dimeric
symmetrical.
bent.
nonpolar.
Which pair is geometrically similar?
a.
b.
c.
d.
SO2 and CO2
CO2 and OF2
PH3 and BF3
SO2 and O3
84.
The bond type and molecular polarity of
SiCl4 are
a.
b.
c.
d.
Bond Type
polar
polar
nonpolar
nonpolar
Polarity of Molecule
nonpolar
polar
polar
nonpolar
�General Chemistry II Sample Test bank
85.
The fact that BCl3 is a planar molecule
while NCl3 is pyramidal can be explained several
different ways. Which is the best rationalization?
a.
b.
c.
d.
86.
a.
b.
c.
d.
Nitrogen is more electronegative than
boron.
The nitrogen atom in NCl3 has a lone pair
of electrons whereas the boron atom in
BCl3 does not.
The nitrogen atom is smaller than the boron
atom
The boron atom in BCl3 is sp3 hybridized,
while the nitrogen atom in NCl3 is sp2
hybridizes
Page 9of 18
isotope?
a.
b.
c.
d.
91.
Which of these molecules is the most
polar? (X and Y are two different elements, Y
being the more electronegative.)
a.
b.
c.
d.
X2
Y2
XYX
XY
X
X
e.
YX
X
The geometry for SeF3+ is
trigonal pyramidal.
tetrahedral.
square planar.
rectangular planar.
92.
The Lewis structure of BrF5 is
F
F
F
Br
F
F
87.
4 days
8 days
12 days
16 days
14
6C
a.
b.
c.
d.
In the beta emission of a species such as
the process may be considered as
the change of a proton into a neutron.
the change of a neutron into a proton.
the same mode of decay as electron capture.
neutrino absorption by the nucleus.
The molecular structure of BrF5 is
a.
b.
c.
d.
88.
square pyramidal.
trigonal pyramidal.
trigonal bipyramidal.
octahedral.
93.
Which compound is a paraffin (methane
series) hydrocarbon?
a.
b.
c.
d.
e.
Which is planar?
a.
b.
c.
d.
e.
PCl3
ClO3CO32NH3
PH3
89.
A molecule of the type ML4 consists of four
bonding pairs of electrons and no lone pairs.
Which structure would it be expected to assume?
a. square planar
b. tetrahedral
c. linear
d. square pyramidal
90.
A sample of a radioactive isotope initially
contains 20 x 1010 atoms. After 16 days, 5 x 1010
atoms remain. What is the half-life of the
C5H12
C5H11OH
(C2H5)2O
C6H6
C6H5Cl
94.
lsobutane differs from butane in that the
former
a.
b.
c.
d.
e.
95.
has a higher molecular weight.
has a different percentage composition.
is not a saturated hydrocarbon.
has a different empirical formula.
has a different structural formula.
Which has the highest boiling point?
a.
b.
c.
d.
n-butane C4H10
n-heptane C7H16
n-hexane C6H14
n-pentane C5H12
�General Chemistry II Sample Test bank
96.
The reaction between acetic acid and ethyl
alcohol is classified as
CH3COOH + C2H5OH " CH3COOC2H5 + H2O
a.
b.
c.
d.
97.
in
a.
b.
c.
d.
Manganese has the oxidation number of +5
[MnF6]3Mn2O7
[MnO4]2[Mn(CN)6]1-
a.
b.
c.
d.
100.
a.
b.
c.
d.
101.
a.
b.
c.
d.
Standard Potentials
Na " Na+ +e
Zn " Zn2++2e
Fe " Fe2+ + 2e
Pb " Pb2++2e
H2 " 2H++ 2e
Cu " Cu2++2e
Hg " Hg2++2e
Ag " Ag+ +e
Eo
2.71 V
0.40 V
0.00 V
-0.80 V
Na+
H2
Cd0
Ag+
In this reaction
3Mg + 2HNO3(dilute) + 6H" 3Mg2+ + 2NO + 4H2O
Standard Potentials
Na" Na+ + e
Al " Al3++3e
Sn2+"Sn4++2e
21 " l2+2e
2F"F2 + 2e
Na+
Al
Sn4+
F2
Eo
2.714 V
1.67 V
-0.14 V
-0.535 V
-2.85 V
104.
Which metal will reduce copper(ll) ions but
not zinc ions?
Which is the strongest oxidizing agent?
Standard Potentials
Na " Na+ + e
Cd " Cd2+ + 2e
H2 " 2H+ + 2e
Ag "Ag+ + e
Na+
O2FMg2+
103.
The greatest oxidizing power (tendency to
gain electrons) is shown by
VO2+
VBr4
NH4VO3
V2(SO4)3
V(CN)63
Cu2+ is oxidized.
Cu2+ gains in oxidation state.
Cu2+ is reduced.
Fe(s) is reduced.
1
2
3
4
6
102.
Which of these isoelectronic ions is the
most polarizable?
a.
b.
c.
d.
Fe(s) + Cu2+ (aq) " Cu(s) + Fe2+ (aq)
Which statement is true for the reaction?
99.
the magnesium acts as a reducing agent. How
many electrons does each magnesium atom
lose?
a.
b.
c.
d.
e.
saponification
addition
esterification
hydrolysis
98.
The highest oxidation number of vanadium
is exhibited in
a.
b.
c.
d.
e.
Page 10of 18
a.
b.
c.
d.
Na
Hg
Pb
Ag
Eo
2.71 V
0.76 V
0.4 V
0.13 V
0.00 V
-0.34.V
-0.85 V
-0.80 V
�General Chemistry II Sample Test bank
105.
A metal, M, forms an oxide of formula
M2O3. The ground state valence shell electron
configuration of the M atom is
a.
b.
c.
d.
ns np
np6
4s13d10
4f7
GaSe
GaSe2
Ga2Se
Ga2Se3
107.
Use this section of a periodic table.
A
E
Q
R
If atoms of R have one d type electron, what
is the formula for a nitride of element A?
a.
b.
c.
d.
A3N
A3N2
AN
AN2
108.
In which pair of particles is the first
member larger than the second member?
a.
b.
c.
d.
2+
Li ; Be
Li+ ; Na+
Li+ ; Li
Be ; Mg
109.
Which would be expected to be the most
electronegative?
a.
b.
c.
d.
111.
In which reaction is the energy term
referred to as the ionization energy?
a.
b.
c.
d.
106.
From your knowledge of the periodic
nature of the elements, what formula would be
anticipated for gallium selenide?
a.
b.
c.
d.
Page 11of 18
P
As
Si
Al
112.
Based on their positions in the periodic
table, which is most likely to replace selenium,
Se, in a biological system?
a. Te
b. Br
c. As
d. I
113.
Which conditions favor the high solubility
of a gas in a liquid?
a. high pressure, high temperature
b. high pressure, low temperature
c. low pressure, high temperature
d. low pressure, low temperature
114.
Ionic compounds in the solid state at room
temperature are generally characterized by their
a. ability to conduct an electric current.
b. high vapor pressures.
c. solubility in polar solvents.
d. solubility in nonpolar solvents.
e. low melting points.
115.
Which precipitate will not dissolve in
aqueous HCl solution?
a. AgBr
b. BaCO3
c. CaSO3
d. Fe(OH)3
e. ZnS
116.
A cellophane bag, which acts as a
membrane permeable only to water, contains a 2
M sugar solution. The bag is immersed in a 1 M
sugar solution. What will happen?
a.
110.
Predict which element would have the
largest difference between its first and second
ionization energies.
a. Sodium
b. Phosphorus
c. Silicon
d. magnesium
NaCl(crystal) + energy " Na+(g) + Cl(g)
Cl(g) + energy "Cl+ + e
Cl(g) + e " Cl(g) + energy
Cl g) + H+(g) " HCl(g) + energy
b.
c.
d.
e.
The bag will soon contain more solution
that will be are concentrated than 2 M.
The bag will soon contain more solution
that will be less concentrated than 2 M.
The bag will lose sugar and the solution in
it will become less concentrated.
The bag will lose water and the solution in
it will become more concentrated.
There will be no change.
�General Chemistry II Sample Test bank
117.
If 0.400 g of a substance R (MW = 80.0
g/mol) is dissolved in 100 g of liquid Q, what is
the molality of the solution?
a.
b.
c.
d.
4.00x103 m
5.00x102 m
5.00 x 103 m
4.00 x 101 m
118.
What is the mole fraction of water in 200. g
of 95% (by mass) ethanol, C2H5OH (mw = 46.0
g/mol)?
a.
b.
c.
d.
0.050
0.12
0.56
0.88
119.
A 0.10 m aqueous solution of HF shows a
freezing point of - 0.198 oC. What is the percent
dissociation of HF? Molal freezing point
constant, Kf for water = 1 .86 oC/m
a.
b.
c.
d.
6.4%
10%
20%
98%
120.
A 0.10 m solution of MgSO4 freezes
at -0.245oC instead of 2 x (- 0.186 oC) as
predicted for ideal behavior. This deviation from
ideality can best be explained on the basis of
a.
b.
c.
d.
interionic attraction.
hydrogen bonding.
the Le Chatelier principle.
the solubility product constant
121.
Which aqueous solution has the smallest
freezing point depression?
a.
b.
c.
d.
0.2 m Ca(NO3)2
0.2 m MgSO4
0.2 m CH3OH
0.2 m K3PO4
122.
When one mole of naphthalene is dissolved
in 1000 g of benzene, the freezing point changes
from 5.51 oC to 0.41 oC. When 20 g of an
unknown organic compound is dissolved in 500 g
of benzene, the freezing point of this solution is
5.00 oC. What is the molar mass of the unknown
organic compound?
Page 12of 18
a.
b.
c.
d.
e.
40 g./mol
200 g/mol
100 g./mol
400 g/mol
128 g./mol
123.
When dilute aqueous solutions of lead(II)
nitrate and potassium bromide are mixed, a
precipitate is observed. The products of this
reaction are
a.
b.
c.
d.
Pb2+(aq) + Br 1- (aq) + KNO3(s)
Br2(aq) + NO2(g) + PbK2(s)
PbO(s) + K+(aq) + Br1-(aq) + NO2(g)
PbBr2(s) + K+(aq) + NO3 1-(aq)
124.
Assuming ideal behavior, what is the vapor
pressure of a solution of 16.0 mol of carbon
tetrachloride and 4.00 mol of dioxane at 23 oC?
Vapor Pressure @ 23 oC
Carbon tetrachloride
100. mm Hg
Dioxane
38.00 mm Hg
a.
b.
c.
d.
50.4 mmHg
62.8 mmHg
74.2 mmHg
87.6 mmHg
125.
What is the empirical formula for the
substance with this analysis: Na=54.0%,
B=8.50%, O=37.5%
a.
b.
c.
d.
Na3BO3
Na4BO4
Na2B2O3
NaB2O2
126.
A hydrocarbon undergoes complete
combustion to give 0.44g of CO2 and 0.27 g of
H2O. What is the simplest (empirical) formula of
the hydrocarbon?
a.
b.
c.
d.
C44H27
CH4
C2H3
CH3
�General Chemistry II Sample Test bank
127.
A 6.80 g coin was dissolved in nitric acid
and 6.21 g of AgCl was precipitated by the
addition of excess sodium chloride, Calculate the
percentage silver in the coin.
Ag+(aq) + Cl1-(aq)" AgCl(s)
a. 24.7%
b. 68.7%
c. 75.3%
d. 91.3%
128.
A 40- mL portion of a 0.1 M MgSO4
solution contains how many grams of MgSO4?
a. 120 g
b. 24 g
c. 0.96 g
d. 0.6 g
e. 0.48 g
129.
An aqueous solution containing 49 g of
sulfuric acid per liter has a concentration of
a. 0.50 M
b. 1.0M
c. 4.9% by mass
d. 4.9M
130.
One hundred milliliters of a solution of
oxalic acid, (COOH)2, is neutralized with 50.0
mL of 0.750 M KOH solution. What is the
molarity of the oxalic acid solution?
a. 0.099 M
b. 0.375 M
c. 0.188 M
d. 0.750 M
e. 0.333 M
131.
How many L of CO2 gas at STP can be
obtained by burning one mole of C3H8?
C3H8(g) + 5 O2(g) "3 CO2(g) + 4H2O(g)
a. 11.2
b. 44.8
c. 67.2
d. 112
132.
What volume of ammonia gas, NH3,
measured at STP, will be produced by the
decomposition of two moles of ammonium
carbonate?
(NH4)2CO3(s) " 2NH3(g) + CO2(g) + H2O(g)
a. 22.4 L
b. 33.6 L
c. 44.8 L
d. 89.6 L
e. 112 L
Page 13of 18
133.
Which change is likely to be accompanied
by the greatest increase in entropy?
a.
b.
c.
d.
N2(g) + 3H2(g) " 2NH3(g) (at 25 oC)
Ag+(aq) + Cl1-(aq) " AgCl(s) (at 25 oC)
CO2(s) " CO2(g) (at - 70 oC)
H2O(g) " H2O(l) (at 100 oC)
134.
When 45.0 g of an alloy at 100.0 oC is
dropped into 100.0 g of water at 25.0 oC the final
temperature is 37.0 oC. What is the specific heat
of the alloy? (for water, specific heat = 4.184 J g1 o -1
C )
a. 0.423 J g-1 oC-1
b. 1.77 J g-1 oC-1
c. 9.88 J g-1 oC-1
d. 48.8 J g-1 oC-1
135.
What is the standard enthalpy of
combustion of C2H6 in kJ mol-1?
Reaction
H2(g) + O2(g) " H2O(l)
C2H4(g) + H2(g) " C2H6(g)
C2H4(g) + 3 O2(g)" 2 CO2(g) + 2
H2O(g)
a.
b.
c.
d.
Ho
-286 kJ
-137 kJ
-1412 kJ
1275 kJ
31561 kJ
1558 kJ
+1834 kJ
136.
Given these equations
Ho = +300 kJ
SO2(g) " O2(g) + S(s)
2SO2(g) + O2(g) " 2SO3(g) Ho = - 200 kJ
calculate the heat of formation of SO3(g).
a. 500 kJ.mol-1
b. +100 kJ.mol-1
c. 400 kJ.mol-1
d. +200 kJ.mol-1
137.
More heat is derived from cooling one
gram of steam at 100 oC to water at 50 oC than
from cooling one gram of liquid water at 100 oC
to 50 oC because
a.
b.
c.
d.
e.
water is a poor thermal conductor.
the steam is hotter than the water.
the steam occupies a greater volume than
the water.
the density of water is greater than that of
steam.
the heat of condensation is evolved.
�General Chemistry II Sample Test bank
138.
Calculate the value of (in kJmol-1) for the
reaction
N2(g) + 3H2(g) "2NH3(g)
Bond Energies
HH
N=N
NH
a.
b.
c.
d.
(kJ. mol-1)
435
946 (in N2)
389
Page 14of 18
142.
Which nuclear equation is properly
balanced?
a. 4He + 9Be " 12C + 1H
b. 4He + 14N " 17O + 1H
c. 4He + 24Mg " 27Si + 1H
d. 14N + 0-1e " 14O
143.
What is the correct reading for the buret?
32
2340 kJ of heat absorbed
213 kJ of heat absorbed
2340 kJ of heat evolved
83 kJ of heat evolved
33
139.
When Al2O3(s) is formed from the elements
at standard conditions, the values of o and Go
at 298 K are -1617 kJ.mol-1 and -1577 kJ.mol-1,
respectively. The standard entropy of formation
per mole, in joules per degree, will be
a. -315
b. -134
c. - 93.3
d. 0.0933
e. +15.7
140.
Vaporization of a liquid is an example of a
process for which
a. H, S, and G are positive at all
temperatures.
b. H and S are positive.
c. G is negative at low temperatures,
positive at high temperatures.
d. H=S
141.
Consider the boiling point of a series of
hydrogen compounds. The abnormally high
boiling point for water is due to
BP
a.
b.
c.
d.
32 mL
32.2 mL
32.26 mL
33.74 mL
144.
A particular chemical reaction has a
negative H and negative S. Which statement is
correct?
a.
b.
c.
d.
The reaction is spontaneous at all
temperatures.
The reaction is nonspontaneous at all
temperatures.
The reaction becomes spontaneous as
temperature increases.
The reaction becomes spontaneous as
temperature decreases.
145.
Which line in the diagram represents the
activation energy for a forward reaction?
a.
b.
c.
d.
A
B
C
D
E
B
Reaction Coordinate
H2O
a.
b.
c.
d.
H2S
H2Se
H2Te
extensive hydrogen bonding
its low dipole moment.
the extreme stability of the compound.
the high electronegativity of hydrogen.
146.
a.
b.
c.
d.
e.
The group C=O is characteristic of
aldehydes.
ketones.
alcohols
esters
acids
�General Chemistry II Sample Test bank
147.
Uranium234 undergoes spontaneous
radioactive decay to give an alpha particle and a
new nucleus, X.
234
U " 4He + X
a. 23090U
b. 23090Th
c. 23894U
d. 23894Pu
148.
Page 15of 18
150.
Consider the phase diagram of a pure
compound. Which statement applies?
What is X?
A
Which is classified as an alkene?
O
B
Temperature
a.
b.
c.
CH3CH2CH
CH3CH2CH3
CH3C = C H
d.
HC
HC
149.
a.
b.
CH2
CH2
c.
CH2
CH2
d.
An example of an organic acid is
O
151.
The path A " C represents sublimation.
Following the path A " B "C the
compound would first liquefy and then
vaporize.
If the compound is in state A, continued
reduction of the pressure (at constant
temperature) will cause it to melt.
None of these statements is correct.
Which is the formula of an alcohol?
a.
b.
CH3CH2CH2 OCH3
CH3CH2CH2CH2 O H
O
a.
CH3CH2COCH3
O
b.
CH3CH2COH
O
c.
CH3 CH2 CCH3
O
c.
CH3CH2CNH2
O
d.
CH3CH2CH2CH
d.
CH3 CH2CH
152.
Which choice best indicates the degree of correctness of this statement? The boiling point of normal
propanol is lower than the boiling point of turpentine.
Temperature
n-propanol
Water
Turpentine
a.
b.
c.
d.
e.
0oC
3.4
4.6
2.1
Vapor Pressure of Substances in mmHg
20oC
50oC
80oC
95oC
100oC
14.5
87.2
376
697
836
17.6
92.0
354.9
760
4.4
17.0
61.3
131.1
The statement is true.
The statement is probably true; additional data would be needed for a final decision.
It is impossible to judge the statement because the data are insufficient.
The statement is probably false; additional data would be needed for a final decision.
The statement is false.
�General Chemistry II Sample Test bank
Page 16of 18
153.
For the reaction 2H2O2 "2H2O +O2 Which plot confirms that the rate is first order with respect to
H2O2?
a.
b.
c.
[H2O2]
1/[H2O2]
[H2O2]2
time
154.
time
d.
log[H2O2]
time
time
Sulfur dioxide can be described by the structures:
S
O
S
O
This implies that
a.
b.
c.
d.
the two bonds in SO2 are of equal length, and the electronic distribution in the two SO bonds is
identical.
the single bond is longer than the double bond and the electronic distribution in the two SO bonds is
different.
an electron pair in the SO2 molecule alternates back and forth between the two sulfur oxygen electron
pairs so that the two different bonds seem to exchange positions.
the SO2 molecule revolves so that the two different bonds seem to exchange positions.
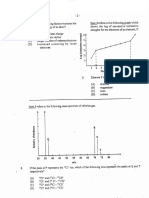
Vapor pressure mm Hg
155.
The graph shows how the vapor pressure of liquid A and of liquid B changes with the temperature.
Select the choice that best indicates the degree of correctness of this statement: The normal boiling point of
liquid B is 78 0C.
1000
900
800
A
600
B
400
300
200
100
0
a.
b.
c.
d.
e.
20 40 60 80
Temperature oC
100
120
The statement is true.
The statement is probably true; additional data would be needed for a final decision.
It is impossible to judge the statement because data are insufficient.
The statement is probably false; additional data would be needed for a final decision.
The statement is false.
�General Chemistry II Sample Test bank
156.
Page 17of 18
The isomerism represented by
H H H
H H
H CCCOH and HCCOCH
H H H
a.
b.
c.
d.
157.
a.
b.
c.
d.
H H
cistrans.
optical.
positional.
geometric.
In a sample of a nearly ideal gas, this graph could represent a plot of
V vs. T at a given constant P.
P vs. T at a given constant V.
P vs. V at a given constant T
PV vs. P at a given constant T
158.
A mixture of 100 g of K2Cr2O7 and 200 g of water is stirred at 60 oC until no more of this salt dissolves.
The resulting solution is decanted (poured off) and cooled to 20 oC. What mass of K2Cr2O7 crystallizes from
the solution during the cooling?
80
60
Solubility
(g/100 mL)
40
20
0
a.
b.
c.
d.
e.
20
40
60
80
Temperature in oC
100
24 g
31 g
43 g
58 g
86 g
159.
The height of the mercury in the right arm open to atmospheric pressure (760 mmHg) is 100 mm and
the height in the left arm is 120 mm.
gas
What is the pressure of the gas in the bulb?
a.
b.
c.
d.
20 mmHg
640 mmHg
740 mmHg
780 mmHg
120 mm
100 mm
�General Chemistry II Sample Test bank
Page 18of 18
Key to Answers
Q#
Ans
Q#
Ans
Q#
Ans
Q#
Ans
Q#
Ans
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
D
B
A
A
D
D
A
A
D
A
D
D
C
D
D
A
C
C
A
A
B
C
D
C
A
A
A
B
D
C
A
B
C
A
B
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
61
62
63
64
65
66
67
68
69
70
E
C
C
C
D
B
B
A
C
B
B
C
A
C
A
A
D
B
D
E
A
D
B
D
D
A
C
D
C
B
D
E
D
B
C
71
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
87
88
89
90
91
92
93
94
95
96
97
98
99
100
101
102
103
104
105
A
C
D
D
B
D
C
C
E
A
D
C
D
A
B
A
A
C
B
B
D
B
A
E
B
C
D
C
C
D
B
B
D
C
A
106
107
108
109
110
111
112
113
114
115
116
117
118
119
120
121
122
123
124
125
126
127
128
129
130
131
132
133
134
135
136
137
138
139
140
D
B
A
A
A
B
A
B
C
A
B
B
B
A
A
C
D
D
D
A
D
B
E
A
C
C
D
C
B
C
C
E
D
B
B
141
142
143
144
145
146
147
148
149
150
151
152
153
154
155
156
157
158
159
A
B
C
D
A
A
B
D
B
D
B
A
D
A
A
C
C
D
C