Professional Documents
Culture Documents
WORKSHOP 9 Addition To Elimination Reactions-3
Uploaded by
Betty Weiss0 ratings0% found this document useful (0 votes)
37 views2 pagesWrite the structures of all the isomeric alkyl bromides having the molecular formula C5H11Br. Which one undergoes E1 elimination at the fastest rate? which one is incapable of reacting by the E2 mechanism? which one yields the most complex mixture of alkenes on E2 elimination?
Original Description:
Original Title
WORKSHOP 9 Addition to Elimination Reactions-3
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentWrite the structures of all the isomeric alkyl bromides having the molecular formula C5H11Br. Which one undergoes E1 elimination at the fastest rate? which one is incapable of reacting by the E2 mechanism? which one yields the most complex mixture of alkenes on E2 elimination?
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
37 views2 pagesWORKSHOP 9 Addition To Elimination Reactions-3
Uploaded by
Betty WeissWrite the structures of all the isomeric alkyl bromides having the molecular formula C5H11Br. Which one undergoes E1 elimination at the fastest rate? which one is incapable of reacting by the E2 mechanism? which one yields the most complex mixture of alkenes on E2 elimination?
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
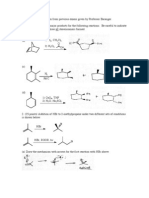
Workshop 9
Alkenes
1. Write the structures of all the isomeric alkyl bromides having the molecular formula C5H11Br
1.
Which one undergoes E1 elimination at the fastest rate?
2. Which one is incapable of reacting by the E2 mechanism?
3. Which ones can yield only a single alkene on E2 elimination?
4. For which isomer does E2 elimination gives two geometrical isomers?
5. Which one yields the most complex mixture of alkenes on E2 elimination?
What are the products of the following reactions under E2 conditions?
A) (4R, 5S) 4-Bromo-4,5-dimethyloctane:
B) (4R, 5R) 4-Bromo-4,5-dimethyloctane:
2. Given the following reaction:
HBr
OH
H2SO4
CH3
heat
a. Predict the product(s) of the reactions
b. Write the mechanism of the reaction(s)
c. Explain every step in the mechanism of the reaction, molecularity, energy of activation
etc.
d. Is the mechanism that you proposed a concerted or stepwise reaction?
e. If you get more than one product, what conclusion can you reach from the mechanism
of the reaction
3. Show how to carry out the following synthetic transformations using any necessary organic
and inorganic reagents. More than one step may be required.
mixture of compounds predicted
major predicted product
You might also like
- Practice For Exam 2Document9 pagesPractice For Exam 2Maria Cecilia Bacani BucasasNo ratings yet
- 12B Alcohol 2Document11 pages12B Alcohol 2Kasun RatnayakeNo ratings yet
- Pranav Question PaperDocument8 pagesPranav Question PaperRohan BandyopadhyayNo ratings yet
- Chem Q.bank Xi 2022Document16 pagesChem Q.bank Xi 2022rishikaa.saxenaNo ratings yet
- Practice Makes Perfect in Chemistry: Organic Chemistry with AnswersFrom EverandPractice Makes Perfect in Chemistry: Organic Chemistry with AnswersNo ratings yet
- Section II Q No. 2. Attempt Any Eight Parts Out of TwelveDocument4 pagesSection II Q No. 2. Attempt Any Eight Parts Out of TwelveUsama IjazNo ratings yet
- CH4103-CH4153 Organic Chemistry 2A - E.O'Reilly M.Zacharska Autumn 2017Document8 pagesCH4103-CH4153 Organic Chemistry 2A - E.O'Reilly M.Zacharska Autumn 2017tadhg.barrett2112No ratings yet
- H.S.C Shri Chandra Tutorials: ChemistryDocument4 pagesH.S.C Shri Chandra Tutorials: ChemistryAmar Kant PandeyNo ratings yet
- Che 232 Test 1 Sptember 2007Document16 pagesChe 232 Test 1 Sptember 2007BONOLO RANKONo ratings yet
- 351 Fin 00Document17 pages351 Fin 00Jaafar SkafiNo ratings yet
- Elimination Reaction - Organic ChemistryDocument8 pagesElimination Reaction - Organic ChemistryreddygrNo ratings yet
- Organic Chemistry 1Document54 pagesOrganic Chemistry 1Nurul Hidayah HamzahNo ratings yet
- Endterm - Training OCIDocument2 pagesEndterm - Training OCIBaga DagaNo ratings yet
- Organic Chemistry Reactions and Mechanisms Identification QuizDocument10 pagesOrganic Chemistry Reactions and Mechanisms Identification QuizudaysrinivasNo ratings yet
- Edexcel - IAS - Organic Chemistry - 1Document27 pagesEdexcel - IAS - Organic Chemistry - 1mostafa barakatNo ratings yet
- Additional Multiple Choice Questions Volume 1Document16 pagesAdditional Multiple Choice Questions Volume 1Andrea DouglasNo ratings yet
- Practice Makes Perfect in Chemistry: Organic ChemistryFrom EverandPractice Makes Perfect in Chemistry: Organic ChemistryRating: 3 out of 5 stars3/5 (1)
- Either X-Ray Diffraction or (Infrared Spectroscopy)Document16 pagesEither X-Ray Diffraction or (Infrared Spectroscopy)Amna HaarisNo ratings yet
- Important Questions of Grade 12 PDFDocument7 pagesImportant Questions of Grade 12 PDFBina NeupaneNo ratings yet
- CH 16 PracticeDocument8 pagesCH 16 Practiced_denbergNo ratings yet
- CHEM 2410 Exam III Practice ExamDocument7 pagesCHEM 2410 Exam III Practice ExamLauren LuLu McCabe0% (1)
- RM 1 Mix QUESTION 1 230418 201559Document7 pagesRM 1 Mix QUESTION 1 230418 201559Jash ShahNo ratings yet
- Practice Makes Perfect in Chemistry: Compounds, Reactions and MolesFrom EverandPractice Makes Perfect in Chemistry: Compounds, Reactions and MolesNo ratings yet
- Edexcel - IAS - Organic Chemistry - 1Document21 pagesEdexcel - IAS - Organic Chemistry - 1mostafa barakatNo ratings yet
- Chapter 5: Structure and Preparation of Alkenes - Elimination ReactionsDocument13 pagesChapter 5: Structure and Preparation of Alkenes - Elimination ReactionsRahma AshrafNo ratings yet
- Exam2 Practice A PDFDocument8 pagesExam2 Practice A PDFĐạt LêNo ratings yet
- ELIMINATIONSDocument10 pagesELIMINATIONSElakkiya shankarNo ratings yet
- !1 Starter AS - Functional GroupsDocument2 pages!1 Starter AS - Functional GroupsTheresa Rebullos BileNo ratings yet
- M.phil. Final Semester - December 2015.CdxDocument5 pagesM.phil. Final Semester - December 2015.CdxbgroyNo ratings yet
- ChemistryDocument9 pagesChemistrySudha NepalNo ratings yet
- Org Chem 5Document48 pagesOrg Chem 5tyron9520100% (1)
- Haloalkanes Test Questions 20aug2023Document3 pagesHaloalkanes Test Questions 20aug2023Robert DanielNo ratings yet
- Class XII chemistry chapter on haloalkanes and haloarenesDocument2 pagesClass XII chemistry chapter on haloalkanes and haloarenesShivank KurmiNo ratings yet
- Chemistry 10Document1 pageChemistry 10Hamza ArshadNo ratings yet
- Design of The Question Paper Chemistry Class - Xii: Time: Three Hours Max. Marks: 70Document16 pagesDesign of The Question Paper Chemistry Class - Xii: Time: Three Hours Max. Marks: 70api-243565143No ratings yet
- CHEM 1315 Exam 3 Practice CDocument7 pagesCHEM 1315 Exam 3 Practice CmikamundkurNo ratings yet
- CBSE 12 Chemistry Question Paper 2010 PDFDocument33 pagesCBSE 12 Chemistry Question Paper 2010 PDFsarvansirNo ratings yet
- SR Inter CHEMISTRY IMP-New With 70% Syllabus-Converted-1Document6 pagesSR Inter CHEMISTRY IMP-New With 70% Syllabus-Converted-1B. SwapnaNo ratings yet
- Class 12 Chemistry Chapter 14 Biomolecules MCQs SolvedDocument19 pagesClass 12 Chemistry Chapter 14 Biomolecules MCQs SolvedTayseer SaudiaNo ratings yet
- CHEM1280 2012 13 Midterm Exam Solution PDFDocument5 pagesCHEM1280 2012 13 Midterm Exam Solution PDFLouisNo ratings yet
- Null 5Document6 pagesNull 5gamerzsilent69No ratings yet
- Tamil Nadu State Board Class XII Chemistry Model PaperDocument9 pagesTamil Nadu State Board Class XII Chemistry Model PaperVishwath RamNo ratings yet
- Questions and Answers - Elimination ReactionDocument17 pagesQuestions and Answers - Elimination ReactionQuốc NguyễnNo ratings yet
- Organic C CCCC CCCCDocument88 pagesOrganic C CCCC CCCCKugan KishurNo ratings yet
- Additional Problems Final Exam Part 2 AnswersDocument10 pagesAdditional Problems Final Exam Part 2 AnswersJohn SmithNo ratings yet
- Prelims Review: Organic Chemistry QuestionsDocument12 pagesPrelims Review: Organic Chemistry QuestionsMarjorie Ann GabuatNo ratings yet
- Lecture 6 - part 2Document13 pagesLecture 6 - part 2rahaf.khalid226No ratings yet
- CHE 232 Test 1 2015 AnsDocument12 pagesCHE 232 Test 1 2015 AnsBONOLO RANKONo ratings yet
- Haloalkanes and HaloarenesDocument1 pageHaloalkanes and HaloarenesKamlesh MauryaNo ratings yet
- Chapter 16Document36 pagesChapter 16aNo ratings yet
- Sr. ChemistryDocument8 pagesSr. ChemistryVeenadhari sai tsalagalla75% (4)
- Revision Test-1, 12th ChemistryDocument4 pagesRevision Test-1, 12th ChemistryVasanthakumar shanmugamNo ratings yet
- BUC Midterm Exams Fall 2011 DetailsDocument10 pagesBUC Midterm Exams Fall 2011 DetailsNeellzz HpNo ratings yet
- Class X BAT 1 - CHEM 2ND 50% SLIP TEST-IIDocument2 pagesClass X BAT 1 - CHEM 2ND 50% SLIP TEST-IIphysicsbooks.storeNo ratings yet
- Halo Alkanes BitsDocument4 pagesHalo Alkanes BitsMonicaNo ratings yet
- 12 Chemistry Aldehydes Ketones and Carboxylic Acids Test 05 PDFDocument1 page12 Chemistry Aldehydes Ketones and Carboxylic Acids Test 05 PDFsiddhartha2862No ratings yet
- Halides and Arenes Chemistry ProblemsDocument7 pagesHalides and Arenes Chemistry ProblemsVishnuNo ratings yet
- Sr. ChemistryDocument8 pagesSr. ChemistryVivek Kandrugula100% (1)
- CHEMISTRY QUESTION PAPERDocument9 pagesCHEMISTRY QUESTION PAPERRishabh JainNo ratings yet
- Chemistry Test - 12th Science-ChemistryDocument7 pagesChemistry Test - 12th Science-ChemistryAishley ChalametNo ratings yet
- Grignard Reaction Lab ReportDocument21 pagesGrignard Reaction Lab ReportBetty WeissNo ratings yet
- Chapter 25Document34 pagesChapter 25Betty Weiss100% (2)
- Aspirin (Asa) Orgo 232 Betty WeissDocument17 pagesAspirin (Asa) Orgo 232 Betty WeissBetty WeissNo ratings yet
- Analytical Chemistry - Mass Spectrometry FragmentsDocument1 pageAnalytical Chemistry - Mass Spectrometry FragmentsBetty WeissNo ratings yet
- Organic Reaction Map v5Document1 pageOrganic Reaction Map v5Betty WeissNo ratings yet
- Chapter 25Document34 pagesChapter 25Betty Weiss100% (2)
- Betty Weiss LAB :DADocument18 pagesBetty Weiss LAB :DABetty WeissNo ratings yet
- Cyclo AlksDocument9 pagesCyclo AlksBetty WeissNo ratings yet
- Benzene Derivatives in Organic ChemistryDocument1 pageBenzene Derivatives in Organic ChemistryAndrea MasonNo ratings yet
- Aspirin (Asa) Orgo 232 Betty WeissDocument17 pagesAspirin (Asa) Orgo 232 Betty WeissBetty WeissNo ratings yet
- Chapter 24Document32 pagesChapter 24Betty Weiss50% (2)
- Remembering Some BasicsDocument34 pagesRemembering Some BasicsBetty WeissNo ratings yet
- Benzene Derivatives in Organic ChemistryDocument1 pageBenzene Derivatives in Organic ChemistryAndrea MasonNo ratings yet
- Organic Functional GroupsDocument1 pageOrganic Functional GroupsCarolina MallamaNo ratings yet
- Types of Organic IsomerismDocument1 pageTypes of Organic IsomerismBetty WeissNo ratings yet
- Chapter 13 Wade 8thDocument80 pagesChapter 13 Wade 8thBetty Weiss60% (5)
- Aspirin (Asa) Orgo 232 Betty WeissDocument17 pagesAspirin (Asa) Orgo 232 Betty WeissBetty WeissNo ratings yet
- Grignard Reaction Lab ReportDocument21 pagesGrignard Reaction Lab ReportBetty WeissNo ratings yet
- Wittigr Reaction SYNT 721Document12 pagesWittigr Reaction SYNT 721Betty Weiss100% (1)