0% found this document useful (0 votes)
35 views1 pageThallium: Toxic Metal and Radiation Detection
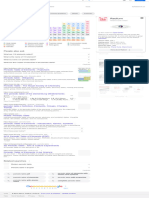
Thallium can be used to detect radiation energy because it is toxic to humans and builds up over time. The periodic table groups elements with similar characteristics, and thallium belongs to the "other metals" group found in the bottom right corner between metalloids and non-metals. This group contains elements like aluminum, gallium, and bismuth that do not belong in other metal groups. Thallium is a soft, bluish-gray metal that is toxic to humans and usually found as an impurity in minerals.
Uploaded by
Rachel Meww CheangCopyright
© Attribution Non-Commercial (BY-NC)
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOC, PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
35 views1 pageThallium: Toxic Metal and Radiation Detection
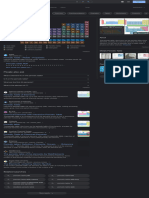
Thallium can be used to detect radiation energy because it is toxic to humans and builds up over time. The periodic table groups elements with similar characteristics, and thallium belongs to the "other metals" group found in the bottom right corner between metalloids and non-metals. This group contains elements like aluminum, gallium, and bismuth that do not belong in other metal groups. Thallium is a soft, bluish-gray metal that is toxic to humans and usually found as an impurity in minerals.
Uploaded by
Rachel Meww CheangCopyright
© Attribution Non-Commercial (BY-NC)
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOC, PDF, TXT or read online on Scribd