Professional Documents
Culture Documents
CHEM Transition Metal Colours
Uploaded by
Ricky LyeOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
CHEM Transition Metal Colours
Uploaded by
Ricky LyeCopyright:
Available Formats
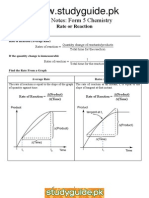
Precipitation reactions of transition metal ions
Cr3+
Colour in aqueous solution Small amount of NaOH Green
Mn2+
Very pale pink
Fe2+
Pale blue-green
Fe3+
Yellow
Ni2+
Emerald green
Cu2+
Blue
Blue-violet ppt Chromium (III) hydroxide, Cr(H2O)3(OH)3
Excess NaOH
Small amount of aqueous NH3
Ppt dissolves to green solution containing [Cr(H2O)2(OH)4]Blue-violet ppt Chromium (III) hydroxide, Cr(H2O)3(OH)3
Gelatinous white ppt Manganese (II) hydroxide, Mn(OH)2, turns brown on standing in air Ppt insoluble in excess
Gelatinous pale green ppt Iron (II) hydroxide, Fe(OH)2, turns brown on standing in air Ppt insoluble in excess
Reddish-brown ppt Iron (III) hydroxide, Fe2O3.xH2O / Fe(H2O)3(OH)3 Ppt insoluble in excess
Emerald green ppt Gelatinous blue Nickel (II) ppt hydroxide, Ni(OH)2 Copper (II) hydroxide, Cu(OH)2
Ppt insoluble in excess
Ppt insoluble in excess
Excess NH3
Ppt dissolves to form yellow solution containing [Cr(NH3)6]3+
Gelatinous white ppt Manganese (II) hydroxide, Mn(OH)2, turns brown on standing in air Ppt insoluble in excess
Gelatinous pale green ppt Iron (II) hydroxide, Fe(OH)2, turns brown on standing in air Ppt insoluble in excess
Reddish-brown ppt Iron (III) hydroxide, Fe2O3.xH2O / Fe(H2O)3(OH)3 Ppt insoluble in excess
Emerald green ppt Gelatinous blue Nickel (II) ppt hydroxide, Ni(OH)2 Copper (II) hydroxide, Cu(OH)2
Ppt dissolves to form lavender blue solution containing [Ni(NH3)6]2+
Ppt dissolves to form deep blue solution containing [Cu(NH3)4(H2O)2]2+
You might also like
- Transition Metal Ion Metal-Aqua Ion With OHDocument2 pagesTransition Metal Ion Metal-Aqua Ion With OHsammam mahdi samiNo ratings yet
- Some Hydroxides Are A: Few Drops of Naoh Xs Naoh Few Drops of NH Xs NH (Conc.) Na Co (Aq) HCL (Conc.)Document1 pageSome Hydroxides Are A: Few Drops of Naoh Xs Naoh Few Drops of NH Xs NH (Conc.) Na Co (Aq) HCL (Conc.)FaridOrahaNo ratings yet
- Transition Metal Ion Metal Aqua Ion With OH With Excess OHDocument1 pageTransition Metal Ion Metal Aqua Ion With OH With Excess OHsammam mahdi samiNo ratings yet
- Redoxcouples 1Document2 pagesRedoxcouples 1api-344570671No ratings yet
- Colour of Ions in Aqueous SolutionDocument2 pagesColour of Ions in Aqueous SolutionLMT_GORDON57% (14)
- AS Level Qualitative AnalysisDocument8 pagesAS Level Qualitative AnalysismahahajNo ratings yet
- Inorganic Compounds in Aqueous Solution - Colours: (Aq) (Aq) (S) (Aq) (S)Document1 pageInorganic Compounds in Aqueous Solution - Colours: (Aq) (Aq) (S) (Aq) (S)daniel1234512345No ratings yet
- Catholic Junior College H2 Chemistry 9729 2019 Practical Handbook - Part 6Document13 pagesCatholic Junior College H2 Chemistry 9729 2019 Practical Handbook - Part 6Timothy HandokoNo ratings yet
- Chemistry Short Notes.Document15 pagesChemistry Short Notes.Mushaa DherreNo ratings yet
- Analysis of Group IIIDocument17 pagesAnalysis of Group IIIRozenNo ratings yet
- Transition Metal Ion and Precipitate ColourDocument4 pagesTransition Metal Ion and Precipitate ColourelezabethNo ratings yet
- Met Al Aqueous Ion Limited Naoh Limited NH Excess Naoh Excess NH Na Co HCLDocument2 pagesMet Al Aqueous Ion Limited Naoh Limited NH Excess Naoh Excess NH Na Co HCLZeenat AfrozeNo ratings yet
- Colors ChemistryDocument4 pagesColors Chemistrykoo heNo ratings yet
- Aqueous Ion ColoursDocument1 pageAqueous Ion ColoursAnita OguniNo ratings yet
- Colours of Inorganic Ions and Complexes Poster PDFDocument1 pageColours of Inorganic Ions and Complexes Poster PDFSophie PriorNo ratings yet
- The p-block elements of groups 15, 16, 17 and 18Document138 pagesThe p-block elements of groups 15, 16, 17 and 18harshadNo ratings yet
- WWW - One School - Net Notes Chemistry SPM Chemistry Formula List Form5Document15 pagesWWW - One School - Net Notes Chemistry SPM Chemistry Formula List Form5Nur AmaleenaNo ratings yet
- D-Block ElementDocument6 pagesD-Block Elementd anjilappaNo ratings yet
- TabelDocument4 pagesTabelayu irsalinaNo ratings yet
- Anion and Cation TestsDocument2 pagesAnion and Cation TestsTanvir Ahmed MazumderNo ratings yet
- Qualitative Analysis of Some IonsDocument42 pagesQualitative Analysis of Some IonsShaina Mae ContilloNo ratings yet
- CP 07 & CP 15 - Analysis of Unknown CompoundsDocument5 pagesCP 07 & CP 15 - Analysis of Unknown Compoundsdameesh9No ratings yet
- 2.6. Reactions of Inorganic Compounds in Aqueous SolutionDocument3 pages2.6. Reactions of Inorganic Compounds in Aqueous Solutionshafiqur rahmanNo ratings yet
- Red/Brown PPT of Purple Solution ofDocument1 pageRed/Brown PPT of Purple Solution ofSusana Natsumy Apaza HuallpaNo ratings yet
- Aqueous Ion Colors: AP Chemistry: Colors Flame Test ColorsDocument1 pageAqueous Ion Colors: AP Chemistry: Colors Flame Test ColorsZhi ZhingNo ratings yet
- Inorganic ChemistryDocument1 pageInorganic ChemistryMihindi WeligamageNo ratings yet
- Identification of Ions and GasesDocument9 pagesIdentification of Ions and GasesAbdullah BilalNo ratings yet
- Practical TesetDocument2 pagesPractical Tesetling chiNo ratings yet
- Flame, Solution, & Gas ColorsDocument2 pagesFlame, Solution, & Gas ColorssharkysharksNo ratings yet
- Stuff I Should Know For The Ap Test But Do Not Know Yet: Ions ListDocument1 pageStuff I Should Know For The Ap Test But Do Not Know Yet: Ions ListShubham MangalNo ratings yet
- Data Sheet Revision PDFDocument2 pagesData Sheet Revision PDFShifa RizwanNo ratings yet
- Chemistry Unit 3B - by Maple Leaf International SchoolDocument36 pagesChemistry Unit 3B - by Maple Leaf International SchoolMohamed Muawwiz Kamil73% (15)
- Learn: ChemistryDocument3 pagesLearn: ChemistryLAKHAN KHANDELWALNo ratings yet
- Notes For Qualitative AnalysisDocument1 pageNotes For Qualitative Analysissatty22No ratings yet
- REDOX REACTIONSDocument4 pagesREDOX REACTIONSajakazNo ratings yet
- 2324 Level M (Gr11 UAE-Gulf) Chemistry Chapter 2 NotesDocument19 pages2324 Level M (Gr11 UAE-Gulf) Chemistry Chapter 2 Notesaminata13536No ratings yet
- SPM Chemistry Formula List Form4Document14 pagesSPM Chemistry Formula List Form4Heng HoweNo ratings yet
- Bab 12 - Nota A+Document7 pagesBab 12 - Nota A+Azemi AhmadNo ratings yet
- TRANSISIDocument61 pagesTRANSISIAlanNo ratings yet
- StuffDocument1 pageStuffrgeahreahNo ratings yet
- Conclusion: Firsh Experiment I: Ion Transition MetalDocument3 pagesConclusion: Firsh Experiment I: Ion Transition Metalayu irsalinaNo ratings yet
- Ligand Substitution and Precipitation ReactionsDocument9 pagesLigand Substitution and Precipitation ReactionsHadia RehmanNo ratings yet
- Oxidation NumberDocument7 pagesOxidation NumberNor Faizahbaizura Abu BakarNo ratings yet
- CH2 Transition Metals Unit V A2 LevelDocument9 pagesCH2 Transition Metals Unit V A2 LevelbillaljavedNo ratings yet
- Colors ListDocument2 pagesColors Listjumajoy35No ratings yet
- Qualitative Analysis Types Reactions NotesDocument3 pagesQualitative Analysis Types Reactions NotesHannieJonnieNo ratings yet
- FORM4CHEMNOTESDocument12 pagesFORM4CHEMNOTESAbbas HaiderNo ratings yet
- Reactions of metal ions in aqueous solution chemistry guideDocument2 pagesReactions of metal ions in aqueous solution chemistry guideSAMANNo ratings yet
- Qualitative Inorganic Analysis GuideDocument17 pagesQualitative Inorganic Analysis GuideDeep AdhiaNo ratings yet
- AnalysisDocument6 pagesAnalysisSifana SohailNo ratings yet
- Potassium (K) : Chapter 14: Reactivity SeriesDocument5 pagesPotassium (K) : Chapter 14: Reactivity SeriesMia PoonNo ratings yet
- Types Of Salts And Their PropertiesDocument6 pagesTypes Of Salts And Their PropertiesSze NingNo ratings yet
- Coordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972From EverandCoordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972A. B. P. LeverNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Handbook of Reagents for Organic Synthesis: Reagents for Heteroarene FunctionalizationFrom EverandHandbook of Reagents for Organic Synthesis: Reagents for Heteroarene FunctionalizationNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersFrom EverandPractice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersNo ratings yet
- Isolation From Chicken Liver and Enzymatic Hydrolysis of GlycogenDocument2 pagesIsolation From Chicken Liver and Enzymatic Hydrolysis of GlycogenKevin Magadia78% (9)
- Introduction To CarbohydratesDocument5 pagesIntroduction To CarbohydratesAdriwayne Francis GonzalesNo ratings yet
- Biograde Organics Products List (VETERINARY INJECTION) .Document10 pagesBiograde Organics Products List (VETERINARY INJECTION) .Puneet GautamNo ratings yet
- Grafilit SL: Properties Appropriate Industries & ApplicationsDocument2 pagesGrafilit SL: Properties Appropriate Industries & ApplicationsFrancisco José Espinosa MásNo ratings yet
- Acid Base Equilibria and StrengthsDocument81 pagesAcid Base Equilibria and StrengthsHooiQIngNo ratings yet
- Lab Rep 6Document4 pagesLab Rep 6Bella LopezNo ratings yet
- Reaction of Alkali Metals With Water and OxygenDocument6 pagesReaction of Alkali Metals With Water and Oxygenみゆ マイクロ100% (1)
- Chem DrawDocument7 pagesChem DrawPutu Ayu WerdhiantyNo ratings yet
- Academic Essay - ExampleDocument2 pagesAcademic Essay - ExampleEvi PartsalidiNo ratings yet
- Bakliwal Tutorials-IIT | p block practice questionsDocument39 pagesBakliwal Tutorials-IIT | p block practice questionsJonathan ParkerNo ratings yet
- CVC Thermoset BrochureDocument28 pagesCVC Thermoset BrochureMattNo ratings yet
- Nomenclature Alkanes Alkenes AlkynesDocument57 pagesNomenclature Alkanes Alkenes AlkynesWilhelm JulioNo ratings yet
- Crude Production & Oil Treatment Facilities TronixDocument84 pagesCrude Production & Oil Treatment Facilities TronixBassem BalghouthiNo ratings yet
- PovidoneDocument2 pagesPovidoneElizabeth WalshNo ratings yet
- Chemical Formulae and EquationsDocument3 pagesChemical Formulae and EquationsFatema KhatunNo ratings yet
- 0607 8 Abstrak Oxo ProcessDocument9 pages0607 8 Abstrak Oxo ProcessMeilyani Farida100% (1)
- AnachemDocument1 pageAnachemPaul Philip LabitoriaNo ratings yet
- Li 2019 Liquid Phase Catalytic Oxidation ofDocument13 pagesLi 2019 Liquid Phase Catalytic Oxidation ofElisabeta StamateNo ratings yet
- KCET 2024 Chemistry Study Plan PDFDocument5 pagesKCET 2024 Chemistry Study Plan PDFshirishgt02No ratings yet
- Synthetic Communications: An International Journal For Rapid Communication of Synthetic Organic ChemistryDocument15 pagesSynthetic Communications: An International Journal For Rapid Communication of Synthetic Organic Chemistrydragon_hsome94No ratings yet
- Unit-4 Water TechnologyDocument22 pagesUnit-4 Water TechnologymaheshkancherlajobNo ratings yet
- Basics of Mining and Mineral ProcessingDocument179 pagesBasics of Mining and Mineral Processingminerales&materiales100% (6)
- Amides: Organic Compounds with Nitrogen AtomsDocument4 pagesAmides: Organic Compounds with Nitrogen AtomsAhmed HammadNo ratings yet
- USP Monographs - Magnesium Oxide CapsulesDocument2 pagesUSP Monographs - Magnesium Oxide CapsulesgirishNo ratings yet
- Cambridge IGCSE: Chemistry 0620/22Document16 pagesCambridge IGCSE: Chemistry 0620/22mostafa barakatNo ratings yet
- Mechanical Properties of Cement Free ConcreteDocument23 pagesMechanical Properties of Cement Free ConcreteKAushik KaRavadiNo ratings yet
- PART A-Answer SchemeDocument4 pagesPART A-Answer SchemeayuNo ratings yet
- COA of Raw MaterialDocument10 pagesCOA of Raw MaterialShafaq ALINo ratings yet
- Exam Style Answers 17 Asal Chem CBDocument2 pagesExam Style Answers 17 Asal Chem CBhxuNo ratings yet