Professional Documents
Culture Documents
Quantum Physics: Black Body Radiation
Uploaded by
smcoolguy68Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Quantum Physics: Black Body Radiation
Uploaded by
smcoolguy68Copyright:
Available Formats
QUANTUM PHYSICS
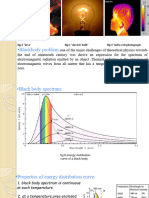
Black Body Radiation A hot body emits thermal radiations which depend on composition and the temperature of the body. The ability of the body to radiate is closely related to its ability to absorb radiation. A Body which is capable of absorbing almost all the radiations incident on it is called a black body. A perfectly black-body can absorb the entire radiations incident on it. Platinum black and Lamp black can absorb almost all the radiations incident on them. Emissive power of a black body: It is defined as the total energy radiated per second from the unit surface area of a black body maintained at certain temperature. Absorptive power of a black body: It is defined as the ratio of the total energy absorbed by the black body to the amount of radiant energy incident on it in a given time interval. The absorptive power of a perfectly black body is 1. Spectral Distribution of energy in thermal radiation (Black Body radiation spectrum) A good absorber of radiation is also a good emitter. Hence when a black body is heated it emits radiations. In practice a black body can be realized with the emission of Ultraviolet, Visible and infrared wavelengths on heating a body. German physicists Lummer and Pringsheim studied the energy density as a function of wavelength for different temperatures of a black body using a spectrograph and a plot is made. This is called Black Body radiation spectrum.
The Salient features of black body radiation spectrum are as below 1) The energy density increases with wavelength then takes a maximum value Em for a particular wavelength m and then decrease to a value zero for longer wavelengths. Hence the Energy distribution in the spectrum is not uniform 2) As the Temperature increases the Wavelength (m) corresponding to the maximum emission energy (Em) shifts towards shorter wavelength side. Thus the m is inversely proportional to temperature (T) and is called Weins Displacement Law. Mathematically . Here b is Weins Constant of value 2.898x10-3 mK.
3) The total energy emitted by the black body at a given temperature is given by the area under the curve and is proportional to the fourth power of temperature. This is called Stefans law of radiation. Mathematically E = T4 here is the Stefans constant of value 5.67 x 10-8 Wm2K-4. Explanation of Black Body Radiation Spectrum Classical Theories Weins Distribution Law: In the year 1893 Wein using thermodynamics showed that the energy emitted per unit volume in the wavelength range and +d
Here C1 and C2 are empirical constants. A suitable selection for these constants helps to explain the experimental curve in the shorter wavelength region. The drawback of this law is it fails to explain the curve in the longer wavelength region. Also according to this equation the energy density at high temperatures tends to zero which contradicts experimental observations.
Rayleigh-Jeans Law: British Physicists Lord Rayleigh and James Jeans made an attempt to explain the Black Body radiation spectrum Based on the concepts formation of standing electromagnetic waves and the law of equipartition of energy. According to this law the energy density of radiation is given by
Here k is Boltzmann constant with value 1.38 x 10-23 JK-1. This law successfully explains the energy distribution of the black body radiation in the longer wavelength region. According to this law black body is expected to radiate large amount of energy in the shorter wavelength region thus leading to no energy available for emission in the longer wavelength region. Experimental observations show that the most of the emissions of the black body radiation occur in the visible and infrared regions. This discrepancy is called Ultraviolet Catastrophe.
Quantum theory of radiation Plancks law of radiation: German physicist Max Plank successfully explained the energy distribution in the black body radiation based on the following assumptions 1) The surface of the black body contains oscillators 2) These oscillators absorb or emit energy in terms of integral multiples of discrete packets called quanta or photons. The energy E of photons is proportional to the frequency of the radiation. Mathematically E=nh here h is a constant called Plancks constant and its value is 6.625 x 10-34 Js, and n can take integer values 3) At thermal equilibrium the rate of absorption and emission of radiation are equal. According to Plancks law of radiation the expression for energy density of radiation is given by
Where c is the velocity of light, k is Boltzmann constant and h is Plancks constant. This law explains the distribution of energy in the black body radiation spectrum completely for all wavelengths and at all temperatures. Also this law can be reduced to Weins distribution law in the shorter wavelength region and to Rayleigh-Jeans law in the longer wavelength region. Deduction of Weins law, Rayleigh-Jeans law from Plancks law
(i) For shorter wavelengths,
is very large
If is very large, i.e.,
is very large
.(1)
Using Plancks equation,
, by substituting equation (1) Where,
(ii) For longer wavelengths,
If is very small, i.e., Now expand We have,
and
is small, is very small
as power series
.....
(Since
is very small, higher power terms could be neglected)
Again using Plancks equation, is reduced to This is known as Rayleigh-Jeans law Photo-Electric effect The emission of electrons from the surface of certain materials when radiation of suitable frequency is incident on it is called the phenomenon of PhotoElectric effect. The electrons emitted are called photo electrons and the material is said to be photo sensitive. This was discovered in the 1887 by Henrich Hertz.
The experimental observations of photoelectric effect are 1) Photo electrons are emitted instantaneously as soon as the radiation is incident 2) Photo electric emission occurs only if the frequency of the incident radiation is greater than a certain value called Threshold frequency. 3) The kinetic energy acquired by photo electrons is directly proportional to the frequency of the incident radiation and is independent of the intensity. 4) The number of photoelectrons emitted depends on the intensity of the incident radiation and is independent of the frequency. Photoelectric effect signifies the particle nature of radiation. Einsteins explanation of the photo electric effect When metal is illuminated with radiation of suitable frequency, the photons of the radiation interact with electrons in the metal. When a photon interacts with an electron, the electron absorbs it and the photon vanishes. The energy acquired by the electron from the photon is made use in two stages. A part of the energy is used by the electron to free itself from the metal since it is bound within metal. Thus some minimum amount of energy is required for the electron just to escape from the metal is called Work Function (). The rest of the energy is carried by the electron as Kinetic Energy (KE). Since the energy of the photon is h the photoelectric effect satisfies the following equation This is called Photoelectric Equation. Here is the frequency of the incident radiation
Here
is the threshold frequency and v is the velocity of electron and m
the mass. Thus from the photoelectric equation, if the frequency of the radiation < no photoelectrons are emitted. Compton Effect The phenomenon of scattering of X-rays from suitable material and hence increase in its wavelength is called Compton Effect.
When X-rays are incident on certain materials they are scattered and the scattered X-rays contain two components. One component has the same wavelength as the incident X-ray and the other with wavelength greater than the incident X-rays. This is due to the scattering of X-ray photons from the electrons present in the material. Due to the transfer of energy from X-ray photon to electron the wavelength of X-ray increases and the electron recoils. This can be treated as collision between two particles. Thus Compton Effect signifies particle nature of radiation. The change in wavelength which is also called Compton Shift is given by
Here is the wavelength of incident X-rays and is the wavelength of scattered Xray is the scattering angle and m0 is the rest mass of electron. The quantity is called Compton Wavelength. Experimental verification A beam of monochromatic X-rays are allowed to fall on a graphite crystal as shown in the figure. The intensity of the scattered X-rays is measure as a function of wavelength of X-rays, at different scattering angles. At each angle, two peaks appear corresponding to scattered X-ray photons with two different wavelengths. The wavelength of one peak does not change as the as the angle is varied. This is called primary or unmodified component. We denote it by . The wavelength of the
other peak varies strongly with the angle and hence it is called modified component. Ii is denoted by . This effect is called Compton effect. The change in wavelength is called Compton shift.
You might also like
- Black Body RadiationDocument12 pagesBlack Body RadiationMahesh Lohith K.S100% (4)
- Quantum Physics Prasanna BPDocument39 pagesQuantum Physics Prasanna BPMukund KnNo ratings yet
- Phy Notes Syl 10Document108 pagesPhy Notes Syl 10vikkumukkaNo ratings yet
- Blackbodyradiation 170319074936Document38 pagesBlackbodyradiation 170319074936LokeshNo ratings yet
- Quantum Mechanics Notes-Part 1Document15 pagesQuantum Mechanics Notes-Part 1aman bhatiaNo ratings yet
- Modern Physics BasicDocument35 pagesModern Physics BasicSGSNo ratings yet
- FALLSEM2022-23 PHY1701 ETH VL2022230106333 Reference Material I 23-09-2022 1black Body RadiationDocument11 pagesFALLSEM2022-23 PHY1701 ETH VL2022230106333 Reference Material I 23-09-2022 1black Body RadiationTanishq AroraNo ratings yet
- Chemistry LN 5 Quantum ChemistryDocument19 pagesChemistry LN 5 Quantum ChemistrySadashiv ChoureNo ratings yet
- AbsorptivityDocument4 pagesAbsorptivityAi VInNo ratings yet
- Quantum - IDocument17 pagesQuantum - I22PCH103 PRAKASH RNo ratings yet
- Modern Physics and Quantum Mechanics Mod-2 PDFDocument28 pagesModern Physics and Quantum Mechanics Mod-2 PDFShreyas SeshadriNo ratings yet
- 3.laws of Radiation and Their Relevance in RemoteDocument20 pages3.laws of Radiation and Their Relevance in RemoteSujata SarkarNo ratings yet
- Study Material/chap.2 Structure and Bonding: Kiita9Document36 pagesStudy Material/chap.2 Structure and Bonding: Kiita9Saurodeep BiswasNo ratings yet
- Unit3 PhyDocument66 pagesUnit3 PhyAk JaNo ratings yet
- Module 5: Modern Physics Lecture 23: Particle and Waves: ObjectivesDocument12 pagesModule 5: Modern Physics Lecture 23: Particle and Waves: ObjectivesDaniyal HashmiNo ratings yet
- UNIT-01: Modern PhysicsDocument28 pagesUNIT-01: Modern PhysicsSree LakshmiNo ratings yet
- High Level Summary On Blackbody RadiationDocument7 pagesHigh Level Summary On Blackbody RadiationMarcela ReynaNo ratings yet
- Quantum Physics Basics FoundationDocument31 pagesQuantum Physics Basics FoundationSebastian mdalahelaNo ratings yet
- Blackbody Radiation and Atomic Emission - Unit - 07Document70 pagesBlackbody Radiation and Atomic Emission - Unit - 07TEBATSONo ratings yet
- 7.L19 L24 Black Body RadiationDocument92 pages7.L19 L24 Black Body RadiationTHE TERMINATORNo ratings yet
- Black Body RadiationDocument9 pagesBlack Body RadiationAnuradha Ramasundar100% (1)
- L21-L27-GPE201-Black Body Radiation-210224Document91 pagesL21-L27-GPE201-Black Body Radiation-210224itzzharry5No ratings yet
- Unit II QuantummechanicsDocument28 pagesUnit II QuantummechanicsKiran ThunuguntlaNo ratings yet
- Bab 2 Blackbody, PhotoelectricDocument35 pagesBab 2 Blackbody, PhotoelectricFakrurazi NajmiNo ratings yet
- Black BodyDocument29 pagesBlack BodySukhwinder Singh GillNo ratings yet
- Satellite Remote SensingDocument52 pagesSatellite Remote SensingAnonymous yjF4yygpPbNo ratings yet
- Module 2 Complete NotesDocument18 pagesModule 2 Complete Noteskumarravi955rNo ratings yet
- 1 Introduction To Quantum Mechanics Unit IDocument12 pages1 Introduction To Quantum Mechanics Unit IyomamaNo ratings yet
- Introduction To Quantum PhysicsDocument74 pagesIntroduction To Quantum PhysicsultimuNo ratings yet
- Physics 1233Document11 pagesPhysics 1233Aayush Singh100% (3)
- QM BasicsDocument12 pagesQM Basicsh.anurag248No ratings yet
- Modern Physics (VTU) 2015-16 PDFDocument12 pagesModern Physics (VTU) 2015-16 PDFU and me SNo ratings yet
- Unit Ii - Quantum Mechanics: D e A D EDocument25 pagesUnit Ii - Quantum Mechanics: D e A D EChiraag ChiruNo ratings yet
- Unit-1 Quantum Mechanics NotesDocument13 pagesUnit-1 Quantum Mechanics NotesPhantom's NetworkNo ratings yet
- Quantum MechanicsDocument5 pagesQuantum MechanicsBADGER I AVINo ratings yet
- Quantum Mechanics Assignment (1) - 1Document10 pagesQuantum Mechanics Assignment (1) - 1sobiabilal4242No ratings yet
- Blackbody RadiationDocument9 pagesBlackbody RadiationTitin Evania ManaluNo ratings yet
- Unit 1 Quantum Mechanics PDFDocument32 pagesUnit 1 Quantum Mechanics PDFDeveshNo ratings yet
- Fallsem2016-17 7570 RM001 14-Jul-2016 Phy1001 EthDocument12 pagesFallsem2016-17 7570 RM001 14-Jul-2016 Phy1001 EthAnirudhNo ratings yet
- Module 3 - Elements of Quantum Mechanics NotesDocument40 pagesModule 3 - Elements of Quantum Mechanics NotesMahek KhushalaniNo ratings yet
- Electromagnetic Waves, Planck's Quantum Theory, and Photoelectric EffectDocument50 pagesElectromagnetic Waves, Planck's Quantum Theory, and Photoelectric EffectElaine MataNo ratings yet
- EMP Lec 1,2Document8 pagesEMP Lec 1,2Prem MurjaniNo ratings yet
- Temu 1Document36 pagesTemu 1Farida UtamiNo ratings yet
- Transport Lab Report Experiment 3Document18 pagesTransport Lab Report Experiment 3nurul nabilah bt khairul anuar100% (2)
- Assgn 1Document4 pagesAssgn 1Spandana PrustyNo ratings yet
- Module 3 PPT - 221124 - 180405Document58 pagesModule 3 PPT - 221124 - 180405Nikhil Rout 22BEC1020No ratings yet
- Chem 242 DR - Salwa M. Al-Rashed: Associate Professor of Physical ChemistryDocument34 pagesChem 242 DR - Salwa M. Al-Rashed: Associate Professor of Physical ChemistryMark Carmelo A AzorNo ratings yet
- Lesson 7.1 Electromagnitic RadiationDocument7 pagesLesson 7.1 Electromagnitic Radiationharpermills.17No ratings yet
- Photons and Matter WavesDocument34 pagesPhotons and Matter Wavesjaymart villartaNo ratings yet
- 02 IntroQuantumPhysicsDocument8 pages02 IntroQuantumPhysicscychan410No ratings yet
- Black Body RadiationDocument33 pagesBlack Body RadiationSafnas Kariapper100% (1)
- HT Assignmet 4 MM19M11Document34 pagesHT Assignmet 4 MM19M11Chetan ChaudhariNo ratings yet
- Unit 1 Quantum MechanicsDocument41 pagesUnit 1 Quantum MechanicsVimala H CNo ratings yet
- GMA220 SUT3 30mar2021 EMR PrinciplesDocument41 pagesGMA220 SUT3 30mar2021 EMR PrinciplesFanelo FelicityNo ratings yet
- Planck'smanual v1Document12 pagesPlanck'smanual v1spyzer.x.001No ratings yet
- QC Lect01 Initial SubjectsDocument20 pagesQC Lect01 Initial SubjectsCosmosianNo ratings yet
- Thermal Radiation: - Classical Theory - Quantum TheoryDocument9 pagesThermal Radiation: - Classical Theory - Quantum TheoryGanantha MarsyafaNo ratings yet
- Thermal Radiation: - Classical Theory - Quantum TheoryDocument9 pagesThermal Radiation: - Classical Theory - Quantum TheoryGanantha MarsyafaNo ratings yet
- Introduction To Quantum PhysicsDocument72 pagesIntroduction To Quantum PhysicsarunsumbriaNo ratings yet
- Unified Field Theory in a Nutshell1: The Quest for the Theory of EverythingFrom EverandUnified Field Theory in a Nutshell1: The Quest for the Theory of EverythingNo ratings yet
- RFP Iecc KochiDocument73 pagesRFP Iecc KochiCUBE ProjectsNo ratings yet
- kx0804 PDFDocument519 pageskx0804 PDFstefan corjuc100% (7)
- DX Service and MaintDocument20 pagesDX Service and MaintCarlos MenaNo ratings yet
- Introduction To MicroWavesDocument5 pagesIntroduction To MicroWavesKrish_666No ratings yet
- Merit-Order Effect of Wind Generation in The Philippines: Is Wind Integration Related To Lower Electricity Spot Market Clearing Price?Document1 pageMerit-Order Effect of Wind Generation in The Philippines: Is Wind Integration Related To Lower Electricity Spot Market Clearing Price?Trei KremmeasNo ratings yet
- 215 D Schema Hydraulique - cssiSImageServletDocument2 pages215 D Schema Hydraulique - cssiSImageServletSieda SiedaNo ratings yet
- Fundamentals of Radiologic Physics Course OutlineDocument7 pagesFundamentals of Radiologic Physics Course OutlineJustin Zeus Operio100% (1)
- W22 Homework #8 AnwsersDocument4 pagesW22 Homework #8 Anwsersiamayesha725No ratings yet
- 2D& 3D-Kate Susannah - Lindsay Sorin and Michelle Maranto-Option 2 - 22.03.23Document11 pages2D& 3D-Kate Susannah - Lindsay Sorin and Michelle Maranto-Option 2 - 22.03.23Miliausha KarimNo ratings yet
- Cho Loss Model Radial TurbineDocument13 pagesCho Loss Model Radial TurbineNicolasNo ratings yet
- Quantum Phase Transitions: Alexander DanielsDocument12 pagesQuantum Phase Transitions: Alexander Danielsapi-288833495No ratings yet
- Plumbing - HVAC August 2010Document48 pagesPlumbing - HVAC August 2010aurelian177100% (1)
- IEEE Power and Energy Magazine Volume 9 Issue 3 2011 (Doi 10.1109/mpe.2011.940579) Katiraei, K.F. Agüero, J.R. - Solar PV Integration ChallengesDocument10 pagesIEEE Power and Energy Magazine Volume 9 Issue 3 2011 (Doi 10.1109/mpe.2011.940579) Katiraei, K.F. Agüero, J.R. - Solar PV Integration Challengesjagadeeshkumar116100% (1)
- 8015-0151-ENTR-41-411-EL-MS-41205 - A0 Method Statement For Cathodic Protection PDFDocument9 pages8015-0151-ENTR-41-411-EL-MS-41205 - A0 Method Statement For Cathodic Protection PDFCripoNo ratings yet
- A Novel High-Gain DC-DC Converter Applied in Fuel Cell VehiclesDocument13 pagesA Novel High-Gain DC-DC Converter Applied in Fuel Cell Vehiclesrock starNo ratings yet
- Sunlight Battery 12V 12ahDocument1 pageSunlight Battery 12V 12ahCarolyn MunozNo ratings yet
- Viking RaidDocument31 pagesViking RaidAnonymous 61kSe1MNo ratings yet
- 12 Chapter 5Document46 pages12 Chapter 5kharisNo ratings yet
- 2 Hydraulic Cylinder EKZDocument25 pages2 Hydraulic Cylinder EKZthanhNo ratings yet
- UHDE - Nitrate Fertilizers PDFDocument24 pagesUHDE - Nitrate Fertilizers PDFvzgscribdNo ratings yet
- How To Avoid Wastage of EnergyDocument1 pageHow To Avoid Wastage of EnergyNazrawi IJobsNo ratings yet
- Distillation Column ModellingDocument1 pageDistillation Column ModellingChem.EnggNo ratings yet
- Dryers: DRYERS - Are Equipment Used For Producing A Dry Solid Product From A Wet Feed General Types of DryersDocument5 pagesDryers: DRYERS - Are Equipment Used For Producing A Dry Solid Product From A Wet Feed General Types of DryersTristan Paul Guerra OrodioNo ratings yet
- Comparison of Various Blade Profiles in A Two-Blade Conventional Savonius Wind TurbineDocument13 pagesComparison of Various Blade Profiles in A Two-Blade Conventional Savonius Wind TurbineSajad MostafaviNo ratings yet
- Air Brakes Module 1Document49 pagesAir Brakes Module 1emreorakNo ratings yet
- Dipen Baroda Follo-Up SheetDocument92 pagesDipen Baroda Follo-Up Sheetanon-272209100% (1)
- PV Cell CharaDocument40 pagesPV Cell CharaBlessy JoyNo ratings yet
- Hns 36W 4P Se: Product DatasheetDocument4 pagesHns 36W 4P Se: Product Datasheetaban Ibis MedicalNo ratings yet
- 1 s2.0 S002236972200364X MainDocument16 pages1 s2.0 S002236972200364X MainFatima NNo ratings yet
- D Aerator SS6000 Liquid Settlement System de Airing Unit SSB0011BDocument1 pageD Aerator SS6000 Liquid Settlement System de Airing Unit SSB0011BMiguel Angel BoldúNo ratings yet